Studies of Single Green Fluorescent Proteins (GFP) in Gels

Figure 1: 100 frames of the emission from an
individual Green Fluorescent Protein molecule (100 ms exposures)
in a polyacrylamide gel, showing the blinking process,
overlaid with the crystal structure of GFP.
Movie: Click here to
see a QuickTime animation (1.1MB) of 100 frames of
the emission from an individual molecule of Green Fluorescent
Protein (100 ms exposures) in a polyacrylamide gel. |
R. M. Dickson, A. Cubitt (Aurora Biosciences), R. Y. Tsien (Howard
Hughes Investigator), and W. E. Moerner
Single molecule studies of individual green fluorescent protein
molecules have yielded the first example of a room temperature
single molecule optical switch.1 A
protein composed of 238 amino acids, GFP folds such that the
chromophore is isolated inside a compact barrel structure. 2,3 Stabilized
and influenced by the three dimensional structure in the immediate
environment of the chromophore, small changes to individual amino
acids can yield large changes in photophysical behavior. By studying
two different red-shifted GFP mutants (T203Y and T203F) which
differ only by the presence of a hydroxyl group near the chromophore,
slight differences in the photophysical properties were observed.
In addition to the differ ences between the mutants, dynamics
only discernable on the single molecule level were observed,
both mutants exhibiting abrupt changes in fluorescence intensity
as a function of time.1 This blinking
behavior likely results from ground state dynamics between at
least two states of the chromophore, only one of which is capable
of being excited at 488 nm and producing fluorescence. Additionally,
a much longer lived state is accessible through excited state
processes. Thermally stable in the dark for many minutes, this
long-lived dark state can be excited at 405 nm to regenerate
the original fluorescent state. 1
Interpretation of the states involved in the blinking and switching
processes are facilitated by the known crystal structures of
wild type3 and red shifted mutant
(S65T) of GFP. 2 The different
absorption peaks observed in these two proteins of known structure
are clearly explained by the observed different chemical states
of the chromophore. Present in the wild type structure, the neutral
form of the chromophore exhibits high energy absorption and emission
peaks. The presence of the anionic chromophore of S65T, on the
other hand, is suggested by long wavelength absorption and emission
peaks. The absorption and emission spectra of the mutants T203Y
and T203F each have two sets of transitions which are indicative
of the two distinct chromophore states being simultaneously accessible.
Interconversion should be possible through a proton transfer
between the chromophore and the surrounding amino acid residues. 4 Since
the optical switching we observed employs wavelengths absorbed
by the neutral and anionic forms of the chromophore, the protein
likely toggles between these two states. Mutation of individual
amino acid residues near the chromophore should allow fine-tuning
of the potential barrier separating the anionic and neutral states,
thus altering their relative ground and excited state stabilities.
The observation of an optical switch at room temperature
in which each molecule is individually addressable and can be
read quasi-nondestructively is an exciting development. Further
single molecule and bulk studies must be performed in order to
better understand the natures of the blinking and switching states.5 Optimization
and control of these processes could lead to new developments
enabling observation of faster protein dynamics within cells.
A well defined starting time would be produced by switching GFP-labeled
proteins within a small focal volume into their fluorescent state;
the subsequent protein dynamics could then be followed. Since
each of these molecules has been shown to be an optical storage
element, long term developments may include storage devices based
on single molecule technology.

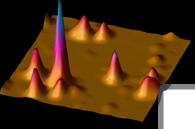
Figure 2: 2.4 micron x 2.4 micron image of individual
Green Fluorescent Protein molecules in a polyacrylamide
gel. |
References
- R. M. Dickson, A. B Cubitt, R. Y. Tsien, and W. E. Moerner, "On/Off
Blinking and Switching Behavior of Single Green Fluorescent
Proteins," Nature, 388, 355 (1997).
- Ormo, M., et al., Science 273, 1392-5 (1996).
- Yang, F., Moss, L.G. & Phillips, J., G. N., Nature Biotech.
14, 1246-51 (1996).
- Brejc, K., et al., Proc. Nat. Acad. Sci., USA 94, 2306-11
(1997).
- W. E. Moerner, "Those Blinking Single Molecules," Science
277, 1059 (1997).
|