Publications
Also you may wish to click below see the PubMed Summary and Google Scholar Summary (ordered by citation count)
 
Journal Covers
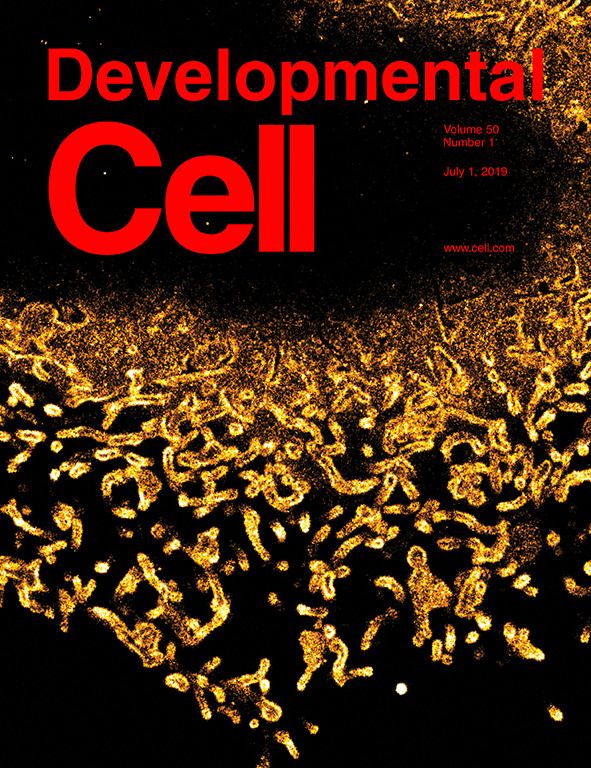 |
COVER: Developmental Cell, 1 July 2019, Vol. 50, No. 1:
The cover shows glycocalyx-decorated membrane tubules on a mammalian cell as reconstructed by super-resolution (SR) microscopy. Using a combination of SR microscopy, image analysis, bioorthogonal chemistry, and transcriptomics analysis, it was possible to extract quantitative information about the height and the nanoscale architecture of the glycocalyx and to monitor its changes during cancer progression.
See Leonhard Möckl*, Kayvon Pedram*, Anish R. Roy, Venkatesh Krishnan, Anna-Karin Gustavsson, Oliver Dorigo, Carolyn R. Bertozzi, W. E. Moerner (*equal contributions) “Quantitative Super-Resolution Microscopy of the Mammalian Glycocalyx,” Dev. Cell 49, article 4559 (2019) (DOI: 10.1016/j.devcell.2019.04.035, published online 16 May 2019). |
 |
 |
COVER: Biophysical Journal, 22 January 2019, Vol. 111, No. 2:
The primary cilium is an important sensory organelle involved in cell signaling and embryonic development in mammals. With a width that spans only a couple hundred nanometers, studying this antenna-like structure in an aqueous environment with high spatial precision requires the use of super-resolution fluorescence microscopy methods. The front cover gives the reader a close look at a surface mesh of the primary cilium, where the color represents how far a point on the mesh is away from the viewer. This image demonstrates the power of combining 3D single-molecule super-resolution microscopy techniques with a surface fitting algorithm, which provides a quantitative framework for studying the unique morphological properties of the primary cilium under various physiological conditions. Artwork by Joshua Yoon.
See Joshua Yoon, Colin J. Comerci, Lucien E. Weiss, Ljiljana Milenkovic, Tim Stearns, and W. E. Moerner, “Revealing the nanoscale morphology of the primary cilium using super-resolution fluorescence microscopy," Biophys. J. 116, 319-329 (2019) (DOI: 10.1016/j.bpj.2018.11.3136, published online 7 December 2018). |
 |
 |
COVER: Optica, 20 November 2015, Vol. 2, No. 11:
The fraction of laser light transmitted through an array of sub-wavelength nanoholes is split into many beams, generating a starburst of fluorescence in a water droplet containing fluorescent dyes. Artwork by Alex von Diezmann and Matthew Lew.
See Alex von Diezmann, Maurice Y. Lee, Matthew D. Lew, and W. E. Moerner, “Correcting field-dependent aberrations with nanoscale accuracy in three-dimensional single-molecule localization microscopy,” Optica 2, 985-993 (2015) (DOI: 10.1364/OPTICA.2.000985, published online November 19, 2015). DOI |
 |
 |
COVER: Nano Letters, 12 September 2013, Vol. 13, No. 9:
An artist's conception of single molecules being examined by an optical microscope (magnifying glass). Single molecules resemble oscillating electric dipoles (orange double-headed arrows) with a toroidal light emission pattern (blue haze around each molecule). If this dipole-like behavior is ignored, there can be significant accuracy errors in single molecule localization-based super-resolution microscopy. The degree of error depends highly on the rotational mobility of the single molecules. Artwork by Matthew Lew.
See Matthew D. Lew*, Mikael P. Backlund*, and W. E. Moerner (*equal contributions), “Rotational Mobility of Single Molecules Affects Localization Accuracy in Super-Resolution Fluorescence Microscopy,” Nano Lett. 13, 3967- 3972 (2013), published online January 29, 2013 and description on these pages |
 |
 |
COVER: Applied Physics Letters, 9 April 2012, Vol. 100, No. 15:
A 3D image of microtubules in a BSC-1 cell acquired by super-resolution fluorescence microscopy is shown in an artistic rendering. The 3D images were recorded using single-molecule active control microscopy (PALM/STORM) on a double-helix point spread function microscope in the Moerner Lab at Stanford University. The coloring is used to show z position. The red double helix emanating from a microscope objective is suggestive of the PSF used in this type of 3D super-resolution microscope. Artwork by Matthew Lew.
See Hsiao-lu D. Lee*, Steffen J. Sahl*, Matthew D. Lew, and W. E. Moerner, “The double-helix microscope super-resolves extended biological structures by localizing single blinking molecules in three dimensions with nanoscale precision,” Appl. Phys. Lett. 100, 153701 (2012), published online 9 April 2012 and description on these pages
|
 |
 |
COVER: Biophysical Journal, 6 April 2011:
Super-resolution fluorescence microscopy of the nucleoid-associated protein HU2 fused to eYFP in the bacteria Caulobacter crescentus far below Abbe's classical diffraction limit. Here we show the gain in resolution by comparing super-resolution fluorescence images (shown inside the camera lens) to conventional diffraction-limited images (shown outside the camera lens) in individual bacterial cells. Spatial positions of single fluorescent proteins derived from eYFP blinking are used not only to generate these images but also to quantify their locations relative to one another using spatial point statistics. The analysis demonstrates that this DNA binding protein displays different amounts of clustering as the cell progresses through its life cycle. Artwork by Steven F. Lee.
See Steven F. Lee, Michael A. Thompson, Monica Schwartz, Lucy Shapiro, and W. E. Moerner, “Super-Resolution Imaging of the Nucleoid-Associated Protein HU in Caulobacter crescentus,” Biophys. J. Lett. 100, L31-L33 (2011) and description on these pages
|
 |
 |
COVER: Journal of Molecular Biology, September 2010:
The cover figure illustrates the conformational changes measured by single-molecule FRET for the mammalian VHL protein during interaction with the prokaryotic chaperonin, GroEL. Unfolded VHL binds to one 7-ring cavity of the dual-cavity GroEL predominantly via two hydrophobic regions, B1 and B2 (white and black circles), causing an initial contraction. Upon GroEL cavity closure by the addition of the cap GroES and ATP, the majority of VHL molecules showing conformational changes experience further contraction between B1 and B2 and expansion between the N- and C-termini. However, a sizable minority of single molecules show the opposite effect, i.e., expansion for B1-B2 and contraction for N-C. This illustrates the stochastic and heterogeneous nature of the chaperonin action on single copies of a non-evolved model substrate protein.
See S. Y. Kim, E. J. Miller, J. Frydman, and W. E. Moerner, J. Molec. Biol. 401, 553-563 (2010), published online 30 June 2010, and description on these pages
|
 |
 |
COVER: Nature Chemistry,
March 2010:
Investigating the dynamics of a single biomolecule typically involves either attaching it to a surface, which may alter its behaviour, or making only fleeting observations in solution before it diffuses away. Now, Goldsmith and Moerner have used a technique that cancels out diffusion to trap single molecules of the photosynthetic antenna protein, allophycocyanin, in solution for over one second. This enables them to measure how the fluorescence of this molecule changes during the course of the observation, as shown conceptually on the cover (image courtesy of Randall Goldsmith and Steven Lee). Cover design by Alex Wing/Nature Chemistry.
See Goldsmith and Moerner, Nature Chem. 2, 179 (2010).
Also see description on these pages |
 |
 |
FRONTISPIECE: Small, October 28, 2009:
The frontispiece illustrates the single fluorophore-bis(micrometer-sized dsDNA) supramolecule. It shows a wide-field single-molecule fluorescence image of the fluorophores in the triblock architectures. The fluorescence appears as white spots, to which, in an artistic view, double-stranded DNA molecules are attached. The triblock supramolecule is synthesized from a tetracarboxylic perylene derivative-bis(oligodeoxynucleotide) by DNA hybridization/ligation and directly imaged by combined scanning force and single-molecule fluorescence microscopy. The synthesis of this type of supramolecule is an important step towards single-molecule electronic devices with reliable contacts after metallization of the DNA strands.
See "Micrometer-Sized DNA-Single Fluorophore-DNA Supramolecule: Synthesis and Single-Molecule Characterization by Lee, Jaeckel, Moerner,and Bao, Small 5 (21), 2418 (2009). |
 |
 |
COVER: ChemPhysChem, July 2003:
The cover picture shows the underlying mechanism and one of the applications of the photorefractive effect, which produces a spatial modulation of the refractive index of a material under nonuniform illumination. As illustrated on the right side, the photorefractive effect begins with light and dark fringes produced by intersecting laser beams, which, in the presence of an applied electric field E0, produce charge separation and eventually a space charge electric field. This field produces a refractive index change, that is, a hologram that can diffract light. A key feature of the effect is that it leads to asymmetric energy transfer between the two beams incident on the material. An application of this effect is image amplification, which is demonstrated on the top left side of the picture where the image of the number 5, carried by a weak beam, is amplified in the presence of a strong beam. Among the best photorefractive materials developed thus far are organic, amorphous glasses, the properties of which depend critically on the glass transition temperature (Tg) and photoconductivity, as well as polarizability anisotropy and hyperpolarizability of the molecules. The picture shows the structure of the photoconductive, nonlinear optical chromophore DCDHF-6 that forms a high-performance low-molecular weight photorefractive glass. |
 |
 |
COVER: Nature Structural Biology, June
1, 2001
A fluorescence
microscope image of kinesin molecules attached to a microtubule
filament. Each kinesin is labeled with a fluorescent dye. The
intensity of the signal (shown in pseudo colors from blue/green
to red/white for low to high intensity) marks the position
of kinesin on the filament. Samples such as the one shown on
the cover were used in single molecule experiments to uncover
a highly flexible state of kinesin when ADP is the nucleotide
bound to the enzyme. [Images: H. Sosa, E. J. G. Peterman, L.
S. B. Goldstein, and W. E. Moerner] |
 |
 |
COVER: Science, May 12, 1999
Optical
images of single molecules from the Moerner Lab. Clockwise from
upper left. Frequency-space: pentacene in p-terphenyl at 2 kelvin
(K). Confocal: protein kinase A regulatory subunit in agarose
gel, 295 K (room temperature). Total internal reflection: green
fluorescent protein in polyacrylamide gel, 295 K. Far-field epifluorescence:
terrylene in p-terphenyl, 2 K. Special section topics begin on
p.1667.[Images: W. E. Moerner, W. P. Ambrose, S. Brasselet, J.
Deich, R. M. Dickson, D. J. Norris, S. S. Taylor] |
|