Nanoscopic Water in Reverse Micelles
Reverse micelles are nanoscopic aggregates made of three components: a water pool, a surfactant, and a nonpolar solvent. A diagram of the general structure is given in Figure 1. The polar heads of the surfactant encapsulate the water pool while the long nonpolar tail extends into the nonpolar solvent. The shape of a reverse micelle is often spherical; however, other shapes like cylinders have been observed.
Figure 1. A diagram of the structure of a spherical reverse micelle.
The AOT/isooctane system produces spherical reverse micelles on average. The size of reverse micelles is related to the amount of water added. The diameter of the water pool in nanometers is given by
dwater = 0.29 * wo + 1.1
where wo is given by
wo = [H2O]/[AOT]
Reverse micelles have been studied using many techniques due to their wide range of uses in medicine, biology, synthesis, and nanotechnology. These aggregates have been used in drug delivery, solubilize biological structures like enzymes and proteins, and catalyze biochemical reactions. They have been used in the formation of different nanoparticles. In synthetic chemistry, reverse micelles are called "microreactors" because they bridge the nonpolar and polar phases.
Across this wide range of applications, the important chemistry occurs at the interface between the water pool and the surfactant. Thus, the properties of the water pool have been studied for many surfactants, nonpolar solvents, and reverse micelle sizes using many experimental and theoretical techniques including using 2D IR in our group. Previous experiment in our group use HOD in H2O as IR probe. Obviously, the advantage is that the probe is the object of interest. And through PSPP and 2D-IR spectroscopies, water molecules inside the medium to large reverse micelle ( wo = 10 and above) can be divided into two parts: the core region, where the water behaves like bulk water, and the shell region, where water dynamics is greatly slowed down by the strong interaction with the surfactant headgroups. The disadvantage, however, is that the short lifetime of OD (~1.8ps) stretch limits the observation window. The CN stretch of SeCN- anion has a strong transition dipole and long vibrational lifetime (~37ps in D2O). We have proved that this anion can probe the water dynamics in bulk water system and we applied this probe in the reverse micelle system. The FTIR spectrum of KSeCN in bulk water and in smallest reverse micelle wo = 1 are very different. (Figure 2) The spectrum in bulk water peaks at 2075 cm-1 and has a significant red wing due to non-Condon effect. The spectrum in wo = 1 reverse micelle, however, is shifted to the blue side at 2065 cm-1 and is symmetric. The diminish of red wing is probably due to the almost negligible hydrogen bonding formed between the anion and water molecules.
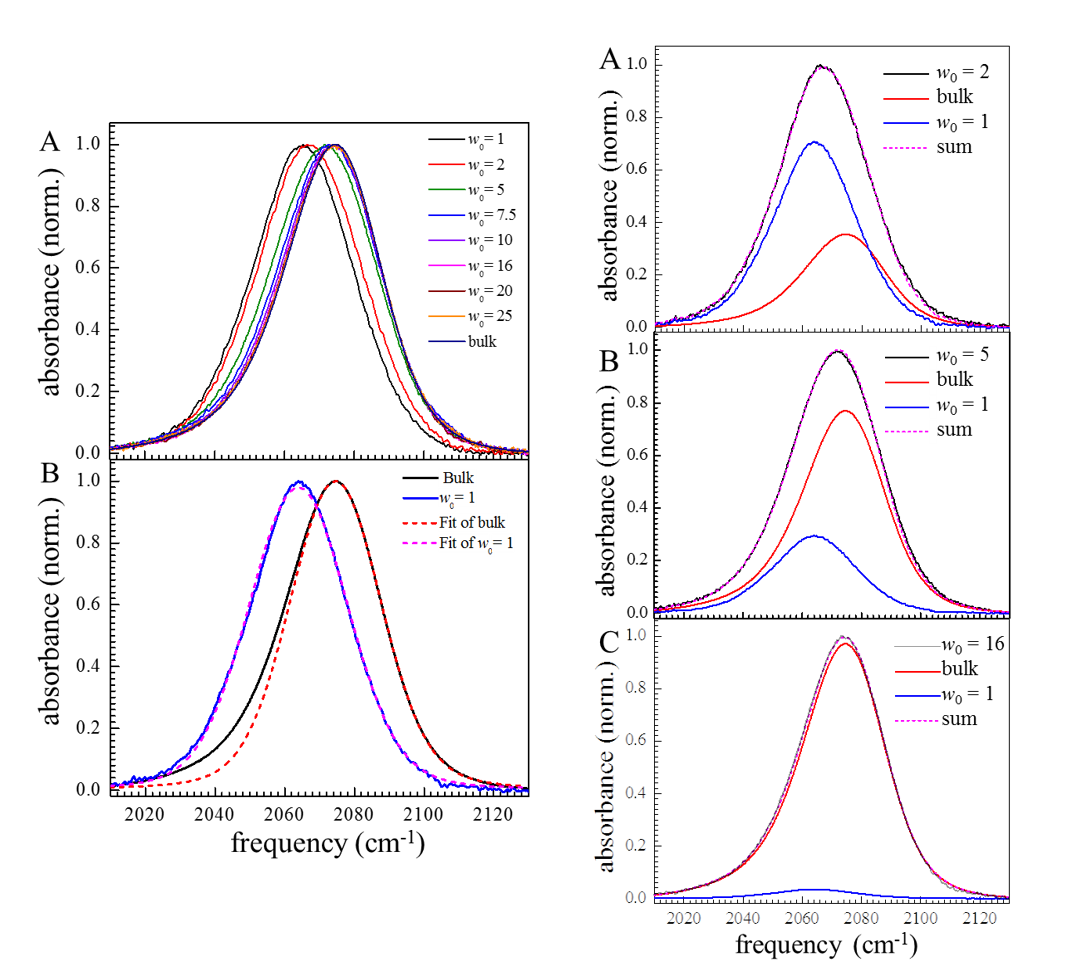
Figure 2. Left: A. KSeCN absorption spectrum in various sizes of AOT reverse micelles. B. The red dashed line is the Voigt fit to the blue part of KSeCN in bulk water, and difference between the black line and red dashed line originates from non-Condon effect duo to hydrogen bonding with water. The pink dashed line is the Voigt fit of KSeCN in wo = 1 reverse micelle, and the disappearance of the red wing indicates that KSeCN in wo = 1 reverse micelles almost has no hydrogen bonding with water molecules.
Right: All of the spectra between wo = 1 RMs and bulk water can be fit by a weighted summation of the wo = 1 spectrum and the bulk water spectrum. A, B and C are examples in wo = 2, 5, and 16 reverse micelles, respectively.
Relevant Publications
364. “Confinement or Properties of the Interface: Dynamics of Nanoscopic
Water in Reverse Micelles,” David E. Moilanen, David B. Spry, Nancy E. Levinger, and M. D. Fayer, J. Am. Chem. Soc. 129 14311-14318 (2007).
386. “Water Dynamics in Large and Small Reverse Micelles: From Two Ensembles to Collective Behavior,” David E. Moilanen, Emily E. Fenn, Daryl Wong, and Michael D. Fayer J. Chem. Phys. 131, 014704 (2009).
388. “Water Dynamics at Neutral and Ionic Interfaces in Reverse Micelles,” Emily E. Fenn, Daryl B. Wong, and M.D. Fayer Proc. Nat. Acad. Sci. U.S.A. 106, 15243-15248 (2009).
444. "Molecular Anion Hydrogen Bonding Dynamics in Aqueous Solution," Rongfeng Yuan, Chang Yan, Amr Tamimi, and Michael D. Fayer J. Phys. Chem. B 119, 13407-13415 (2015).
462. "Dynamics in a Water Interfacial Boundary Layer Investigated with IR Polarization Selective Pump-probe Experiments," Rongfeng Yuan, Chang Yan, Jun Nishida, Michael D. Fayer J. Phys. Chem. B 121, 4530-4537 (2017).
