

|
 |
 |

|
 |
|

|

|
 |
|
 |
Molecular Biology
Infection and Replication
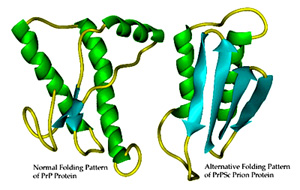
Prions are proteins. The body encodes a gene for a normal protein, PrP, usually found in lymphocytes and CNS neurons. PrP has a normal conformation known as PrPc, which is genetically encoded. It becomes misfolded into a form known as PrPsc, and causes other proteins to become misfolded as well.
 |
| http://www.millerandlevine.com/news/bse/image/prions.jpg |
The protein goes from a 40% alpha helix with almost no beta sheet to 30% alpha helix and 45% beta sheet but retains same amino acid sequence. It had previously been thought that the amino acid sequence could have mainly one active structure, but this shows that's not true.
As opposed to viruses, where certain strains have specific structures due to their genomes, the characteristics of the prion reflect the chromosomal structure of the PrP gene belonging to whichever species it just replicated in. This is thought to be encoded in the tertiary structure of the protein.
Unlike PrPc, the misfolded PrPsc is not easily digested by proteases. The abnormal protein can't be broken down in the body and so it aggregates in the brain. It does not cause an immune response.
There are three types of prion diseases: spontaneous, acquired and genetic. The cause for spontaneous is unknown. Acquired is caused by, in humans, ingestion of infected tissue. If the PrP gene mutates it can cause genetically inherited disease. There are 20 mutations that can cause these diseases and 5 are significantly genetically linked. The mutations have been found all through the protein; there is not just one area that mutates to cause prion diseases.