Multiple Wnt receptors
Reviews by Jiang and Cong 2016 and Lehoczky and Tabin 2018
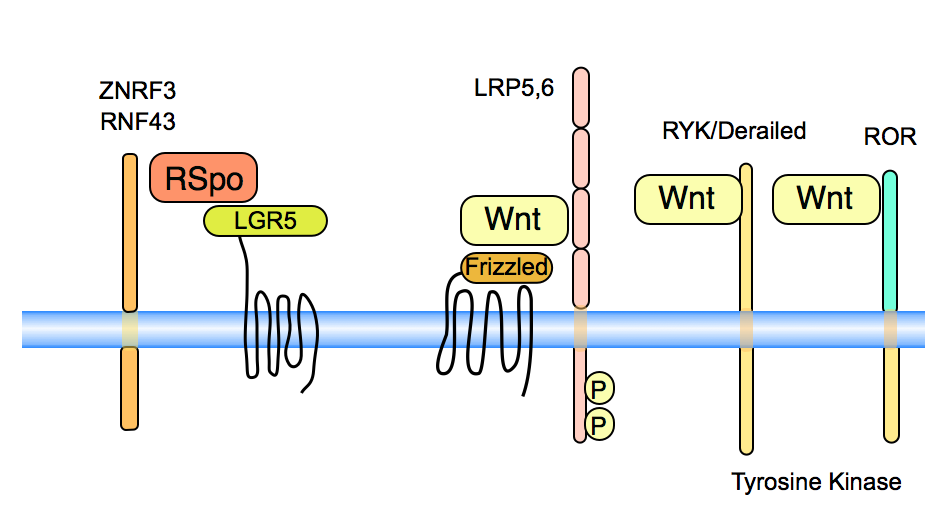
Frizzled proteins are seven-transmembrane proteins that act as the primary receptors for Wnt signals. Wnts can bind the the CRD (cysteine-rich domain) of Frizzled. The structure of Wnt as bound to its receptor Frizzled shows that the lipid, sticking out of the Wnt protein, inserts into a hydrophobic cavity in Frizzled (Janda et al, 2012).
LRPs and Arrow in Drosophila are long single-pass transmembrane proteins. Arrow is genetically required for Wingless signaling (Wehrli, 2000) and mouse LRP mutations are similar in phenotype to Wnt mutants (Pinson, 2000).
Beyond the well known receptors Frizzled and LRP, there are several other transmembrane proteins that act as specific receptors for Wnt proteins or the Wnt agonists, such as R-Spondin. In several cases, these receptors play a role in alternative Wnt pathways. See MacDonald and He (2012), Niehrs (2012) and van Amerongen et al (2008) for reviews.
R-spondins interact on the cell surface with members of the LGR5 family, to enhance Wnt signaling (Glinka et al, 2011, Carmon et al, 2011, De Lau et al, 2011).
ZNRF3 and RNF3 are transmembrane molecules that down-regulate Wnt signaling. They have E3 ubiquitin ligase activity acting on the Frizzled molecules, leading to turn-over of these receptors (Hao et al, 2012, Koo et al, 2012). Binding of R-Spondin to ZNRF3 has been postulated to down-regulate the activity of the ZNRF3 activity, thereby enhancing Wnt signaling as the Frizzled receptors now become available (Hao et al, 2012). ZNRF3 and RNF3 are mutated in some tumors (Koo et al, 2012).
It should be noted though that Szenker-Ravi et al, (2018) have obtained results indicating that RSPO2, without the LGR4/5/6 receptors, serves as a direct antagonistic ligand to RNF43 and ZNRF3. They established that the triple and ubiquitous knockout of Lgr4, Lgr5 and Lgr6 in mice did not recapitulate the known Rspo2 or Rspo3 loss-of-function phenotypes. Moreover, based on cell culture and biochemical experiments, Lebensohn and Rohatgi (2018) have shown that R-spondins can potentiate WNT signaling without LGRs. Reviewed by Lehoczky and Tabin 2018
The Derailed/RYK proteins are transmembrane tyrosine kinases. They have a Wnt binding domain similar to the WIF proteins, a group of secreted Wnt binding factors (Hsieh 1999). In Drosophila, Derailed interacts with DWnt5 to mediate axon guidance, Yoshikawa 2003) and in salivary gland migration (Harris, 2007). There is also evidence in C.elegans (Lin-18, Inoue 2004) and in mammals (RYK, Lu et al 2004) for Wnt interaction with these receptors. RYK can interact with Dsh ( Lu et al 2004). and function in Wnt/beta-catenin/TCF signaling but in other contexts, these pathways are not linked.
The ROR transmembrane tyrosine kinases can also bind to Wnts, using a CRD motif similar to that of the Frizzleds (Kani et al, 2004, Mikels 2006) This interaction is conserved in evolution, also found in C. elegans (Forrester, 2004). Wnt5a can specifically bind to Ror2 and thereby inhibit Wnt/beta-catenin/TCF signaling (Mikels 2006). The mechanism of inhibition is not clear however. Evidence in Xenopus indicates that Wnt5a signals through Ror2 to control expression of cadherins and convergent extension (Schambony 2007). Loss of both ROR1 and ROR2 leads to defects, including decreased branching of sympathetic neuron axons and major defects in aspects of embryonic development. These processes are dependent upon morphogenetic movements, such as and loss of ROR signaling leads to truncation of the caudal axis, the limbs, and facial structures. These phenotypes mirror phenotypes observed in Wnt5a null mutant mice (Ho et al, 2012). Interestingy, the human disease Robinow syndrome is associated with mutations in three different Wnt signaling components: ROR2 (Van Bokhoven et al, 2000) , WNT5A and DVL1.
Two other receptors, GPR 124 and RECK, are required for correct Wnt signaling in establishing the blood brain barrier (Zhou and Nathans, 2014; Posokhova et al., 2015; Eubelen et al, 2018) Here, Wnt7 is a locally acting ligand, working through FZD4 and LRP5. In this complex, Wnt7A can interact with the RECK molecule as well (Eubelen et al, 2018). Reviewed in Junge, HJ Neuron 2017 Aug 30;95(5):983-985. doi: 10.1016/j.neuron.2017.08.026.
From these and other data, a picture emerges of various Wnts binding to different classes of receptors. The outcome of signaling is then regulated by receptor context and is not intrinsic to a particular Wnt. Wnt5a for example can signal to the Wnt/beta-catenin/TCF pathway when interacting with Frizzled4 and LRP5 (Mikels 2006), as well as through ROR2. Wnt11, long viewed as a "non-canonical" Wnt is clearly also able to signal via beta-catenin in a Frizzled-FRL dependent manner (Tao et al , 2005).
It should also be mentioned that receptor context plays a role in the distinction between Frizzled-mediated planar polarity signaling and Wnt/beta-catenin signaling in Drosophila. The Wnt co-receptor Arrow is genetically necessary beta-catenin (armadillo) signaling, but is dispensable for planar polarity (Wehrli, 2000).