LRP/Arrow
LRPs and Arrow in Drosophila are long single-pass transmembrane proteins. Arrow is genetically required for Wingless signaling (Wehrli, 2000) and mouse LRP mutations are similar in phenotype to Wnt mutants (Pinson, 2000). In Xenopus, overexpression of LRP can activate Wnt signaling (Tamai, 2000). There is evidence that Wnts can bind directly to the extra-cellular domain of LRP and form a ternary complex with the Frizzled receptor (Tamai, 2000) although this is not confirmed by Mao et al, 2001 or by Wu and Nusse, 2002. The cytoplasmic domain of LRP may interact with Axin (Mao, 2001; Tolwinski 2003); Axin binds selectively to a phosphorylated domain on LRP (Tamai 2004). Phosphorylation of the tail of LRP is regulated by two protein kinases: GSK3 and CK1gamma (Zeng, 2005; Davidson 2005; reviewed by Nusse, 2005
LRPs can bind directly to the Wnt inhibitor Dickkopf (Mao, 2001; Bafico, 2001, Semenov, 2001; reviewed in Nusse, 2001). Wise and the related protein SOST/Sclerostin can also bind to LRP, to either inhibit or stimulate Wnt signaling (Itasaki, 2003; Semenov 2005, Li 2005)
Wei et al (2006) have shown that LRP6 can mediate Internalization and Lethality of Anthrax Toxin.
There are 2 LRPs that can act in Wnt signaling. Interestingly, mutations in LRP 5 in humans affect bone and eye development (reviewed in Patel and Karsenty, 2002). LRP5 is specific for Wnt5a signaling (with Frizzled4) to activate Wnt reporters (Mikels, 2006). Mutant forms of LRP 5 are implicated in Hyperparathyroidism (Bjorklund, 2007)
The boca/mesd protein in specifically involved in LRP5/6 transport and mutations in this gene in Drosophila and mice have Wnt specific phenotypes (Hsieh, 2003, Culi, 2003)
Human Gene |
Human phenotype |
Location |
Mouse gene |
Location |
Mouse phenotype |
|---|---|---|---|---|---|
| LRP5 | Loss of function: low bone density Gong,
2001
Gain of function: high bone density (Little, 2002, Boyden, 2002) Loss of function Familial Exudative Vitreoretinopathy (Toomes et al, 2004) |
11q13.4 | Lrp5 | 19 | decrease in osteoblast proliferation, osteopenia,
and persistent embryonic eye vascularization (Kato
M, 2002)
Defects in cholesterol metabolism and glucose-induced insulin secretion (Fujino, 2002) Double LRP5,6 mutant: gastrulation defect, no primitive streak (Kelly, 2004) deficits in BMD and limb formation in double mutant LRP5,6 (Holmen 2004) reduced cell death and vascular regression failure (Lobov 2005) (also in LEF1 and wnt7B mutants) Activates Wnt reporter with Wnt5A and Fz4 (Mikels, 2006) Bone formation (through serotonin synthesis) Yadav 2008 |
| LRP6 | Autosomal-Dominant Oligodontia (Massink, 2015) | 12p11-p13 | Lrp6 | 6 | developmental defects similar to mutations in
individual Wnt genes (Pinson,
2000).
Defects in dorsal thalamic development (Zhou et al, 2004) spontaneous hypomorphic mutation ringelschwanz. skeletal, neural tube abnormalities (Kokubu, 2004) Double LRP5,6 mutant: gastrulation defect, no primitive streak (Kelly, 2004) deficits in BMD and limb formation in double mutant LRP5,6 (Holmen 2004) hypoplasia of the developing neocortex Zhou, 2006 |
| Drosophila Arrow | . | 50A11 Arrow in Flybase | . | . | . |
Schematic structure Arrow/LRP (Figure made by Michael Povelones)
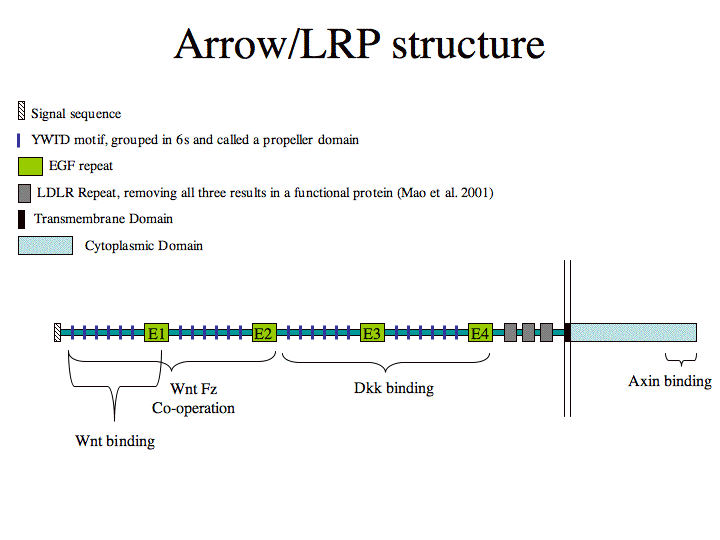
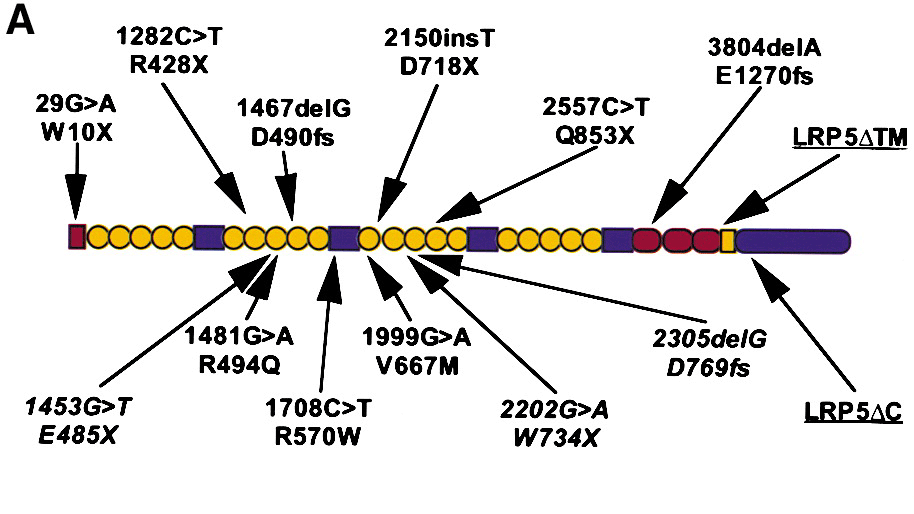
Figure from Gong et al, (Cell 107, 513, 2001)
The predicted structure of wild-type LRP5. LRP5 contains a 29 amino acid signal peptide (red rectangle), 20 YWTD spacer repeat domains (yellow circles) interspersed by four EGF-like domains (blue rectangles), three LDL receptor-like ligand binding domains (red ovals), a transmembrane domain (yellow rectangle), and a 200 amino acid residue cytoplasmic domain (blue oval). Six disease-causing, homozygous frameshift and nonsense mutations and their predicted effects upon the polypeptide sequence are shown above the diagram. Putative disease-causing homozygous missense mutations are shown below the diagram, as are heterozygous mutations (in italics) found in affected individuals from nonconsanguineous unions.