Photovoltaic Retinal Prosthesis for Restoration of Sight in Retinal Degeneration
Retinal degenerative diseases lead to blindness due to loss of the “image capturing” photoreceptors, while neurons in the “image-processing” inner retinal layers are relatively well preserved. Information can be reintroduced into the visual system using electrical stimulation of the second-order retinal neurons, the bipolar cells, which then transfer their responses to the rest of the retinal neural network. This approach enables preservation of many features of the retinal signal processing, and thereby allows restoration of sight. We developed a photovoltaic subretinal prosthesis, which converts incident light into pulsed electric current, stimulating the nearby inner retinal neurons. Results of the clinical trial with our implants (PRIMA, Pixium Vision) having 100μm pixels, as well as preclinical measurements in rodents with 75 and 55 μm pixels, confirm that spatial resolution of prosthetic vision can reach the sampling density limit.
For a broad acceptance of this technology by patients who lost central vision due to age-related macular degeneration, visual acuity should exceed 20/100, which requires pixels smaller than 25um. Radial expansion of electric field in front of the flat arrays precludes scaling the pixels to such small dimensions. We are working on 3-dimensional electro-neural interfaces which should enable such a high resolution, and may even reach single-cell selectivity.
1-min animation of the concept
3-min animation and summary from the Department of Ophthalmology
Recent seminar about the project (March 8, 2024)
System Design
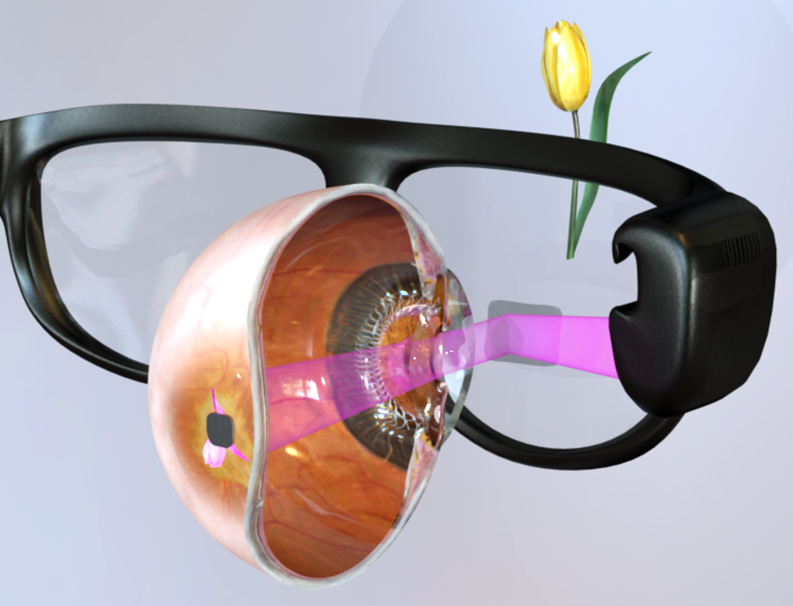 The data stream from a video camera is processed by a pocket PC, and the resulting images are displayed on the augmented-reality glasses, shown on the right. Images are projected from a DMD microdisplay onto the subretinal implant (lower image on the right) using pulsed (1-10 ms) near-infrared (880 nm) light. These light pulses are photovoltaicly converted into bi-phasic pulses of electric current flowing through the retina between the active and return electrode in each pixel, which stimulate the nearby inner retinal neurons, thereby introducing visual information into the retinal neural network. Optical delivery of the information and power allows for simultaneous activation of thousands of pixels in the implant, and retains the natural link between the eye movements and visual perception.
The data stream from a video camera is processed by a pocket PC, and the resulting images are displayed on the augmented-reality glasses, shown on the right. Images are projected from a DMD microdisplay onto the subretinal implant (lower image on the right) using pulsed (1-10 ms) near-infrared (880 nm) light. These light pulses are photovoltaicly converted into bi-phasic pulses of electric current flowing through the retina between the active and return electrode in each pixel, which stimulate the nearby inner retinal neurons, thereby introducing visual information into the retinal neural network. Optical delivery of the information and power allows for simultaneous activation of thousands of pixels in the implant, and retains the natural link between the eye movements and visual perception.
 In preclinical studies, we found that prosthetic vision with subretinal implants preserves many features of the natural visual processing, including flicker fusion at high frequencies (>20 Hz), adaptation to static images, antagonistic center-surround organization and non-linear summation of subunits in the receptive fields, providing high spatial resolution. Results of the clinical trial with our implants (PRIMA, Pixium Vision) having 100μm pixels confirm that spatial resolution of prosthetic vision can reach the pixel pitch (20/420 visual acuity with 100μm pixels). Patients also demonstrated simultaneous perception of the peripheral natural and the central prosthetic vision.
In preclinical studies, we found that prosthetic vision with subretinal implants preserves many features of the natural visual processing, including flicker fusion at high frequencies (>20 Hz), adaptation to static images, antagonistic center-surround organization and non-linear summation of subunits in the receptive fields, providing high spatial resolution. Results of the clinical trial with our implants (PRIMA, Pixium Vision) having 100μm pixels confirm that spatial resolution of prosthetic vision can reach the pixel pitch (20/420 visual acuity with 100μm pixels). Patients also demonstrated simultaneous perception of the peripheral natural and the central prosthetic vision.
We continue to study the mechanisms of neural stimulation and characteristics of prosthetic vision ex-vivio and in-vivo, and optimize the system to enable high resolution prosthetic vision. These studies include modeling of the electric field in tissue, neural response to electric field, electrode-electrolyte interface and circuit dynamics, fabrication of the implants, and electrophysiological assessement of the retinal, cortical and behavioral responses to visual stimuli. We also participate in the design and data analys of the clinical trials of our PRIMA system manufactured by Pixium Vision.
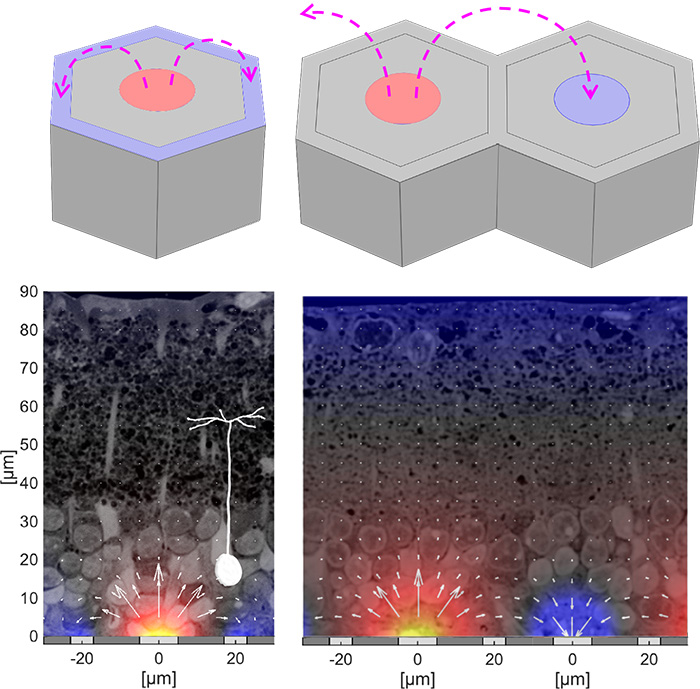
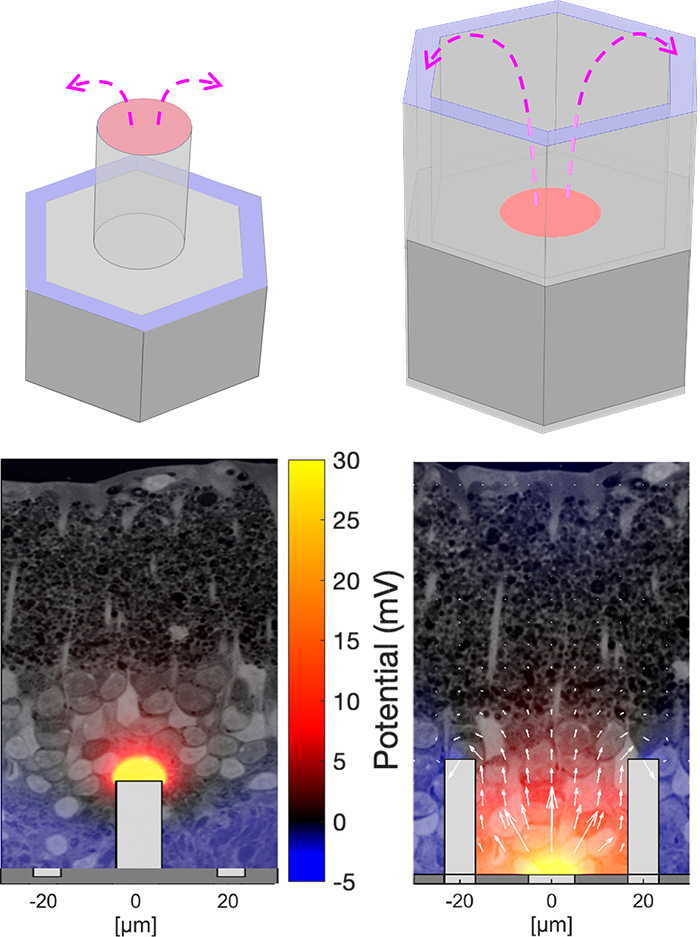
Field shaping and 3-dimensional electro-neural interfaceFor a broad acceptance of this technology by patients with geographic atrophy, visual acuity should exceed 20/100, which requires pixels smaller than 25 μm. However, with small pixels, local return electrodes, typically used for crosstalk suppression, result in overly constrained penetration of electric field into the retina, which impedes the neural stimulation and limits the pixels miniaturization (left image). We are working on two approaches to more efficient field shaping with smaller pixels: (a) optical current steering, where pixels can be used transiently either as anodes or as cathodes, and (b) 3-dimensional electrodes for enhanced penetration of electric field into the retina. The current steering approach (image to the right) is based on the fact that charge accumulation at the electrodes capacitively coupled to the electrolyte, as well as the electric potential generated by the neighboring electrodes in electrolyte, elevate the voltage across the p-n junction in a photovoltaic pixel. Conductance of the diode exponentially increases with the forward bias, effectively transforming the active electrode into a transient return when the potential is above the turn-on voltage of the diode. By optically pre-charging the dark pixels prior to the stimulation pulse, the current sunk via the dark pixels (serving as cathodes) suppresses the crosstalk caused by the electric field spreading from the neighboring anodes (bottom image). Pixel cunductance can be further increased (and stabilized) using a shunt resistor in parallel with the photodiode. With 40μm pixels and the optical current steering, prosthetic grating acuity was found to be 34 μm, matching the row pitch in a hexagonal array. With 20 μm pixels, the prosthetic resolution was found to be 27 μm, matching the natural resolution of 28μm in LE rats. Since the retinal resolution in humans is about 5 μm, with 20 μm pixels, prosthetic visual acuity may reach 20/80, which would provide very meaningful restoration of sight for patients who lost central vision due to age-related macular degeneration. The 3-D electrodes in subretinal space can provide closer proximity to the target neurons utilizing the effect of cellular migration into the voids in subretinal space. Cells migrating into the honeycombs with active electrode (red) at the bottom and return (blue) electrode at the top, are exposed to nearly vertical electric field (right image). Since this field orientation matches the vertical alignment of bipolar cells, stimulation threshold is reduced and it is decoupled from the pixel width, thereby enabling scaling the pixel size down to cellular dimensions. Alternatively, pillar electrodes can be used - due to cellular migration into the voids, pillar electrodes penetrate closer to the inner nuclear layer, where bipolar cells reside, thereby reducing the stimulation threshold (left image). |
|