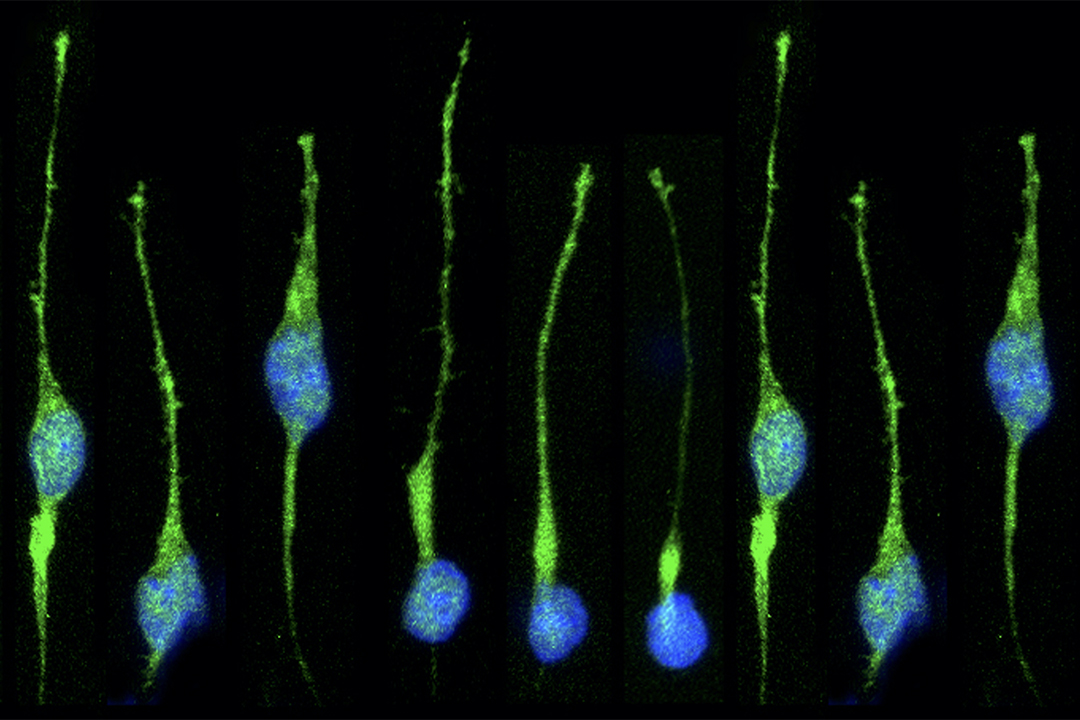
The use of genetics to study the development of the telencephalon and derivatives such as the cerebral cortex was quite limited many years ago, when we started working in this area. The telencephalon begins to form midway through gestation, and targeted mutations in genes suspected of playing roles in its development often lead to early phenotypes that preclude analysis of their role at later stages. This problem can be circumvented using a Cre/loxP recombination system. We generated a mouse line was produced in which Cre was targeted to the Foxg1 (BF-1) locus, a gene expressed specifically in the telencephalon and discrete head structures. Crosses between Foxg1-Cre mice and three separate loxP reporter mice generated embryos with recombination patterns matching that expected from the normal pattern of Foxg1 expression. Recombination occurs invariably in the telencephalon, anterior optic vesicle, otic vesicle, facial and head ectoderm, olfactory epithelium, mid-hindbrain junction, and pharyngeal pouches. Recombination in some animals also occurs less efficiently in tissues not known to express Foxg1. Both the genetic background of the parental mice and the loxP target allele can each contribute to differences in the exact pattern of recombination. Overall, Foxg1-Cre mice are useful for the deletion of floxed target genes in a Foxg1-like pattern. Several signaling molecules, including Sonic Hedgehog (SHH), Fibroblast Growth Factor 8 (FGF8), and Bone Morphogenetic Proteins (BMPs), have been implicated in patterning the developing forebrain, and we have used a conditional genetic approach to tease apart the roles of these molecules during development.
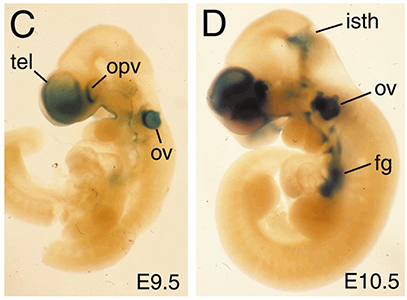
BMP signaling has been hypothesized to pattern the dorsal midline of the forebrain by acting in a concentration-dependent manner, with different levels of BMPs inducing distinct cell fates. Mice lacking Bmp4 do not exhibit obvious changes in midline patterning (Hébert et al., 2003), but other BMP ligands are also present in this region. We crossed Foxg1-Cre mice to mice that carry a floxed Bmpr1a allele and found that BMP signaling is compromised throughout the dorsal telencephalon, enabling us to ascertain whether specific levels of BMP signaling induce discrete fates (Hébert et al, 2002). In these mice, only choroid plexus cells fail to be specified or differentiate, and instead remain proliferative. These cells do not adopt the fate of their neighboring telencephalic cells in the cortical hem, yet they continue to express dorsal midline markers. These results demonstrate that BMP signaling is required for the formation of the most dorsal telencephalic derivative, the choroid plexus, and provide evidence that BMP signaling plays an essential role in locally patterning the dorsal midline, but fail to support a more global, concentration-dependent role in specifying telencephalic cell fates. In subsequent studies, performed primarily in the Hébert lab at Albert Einstein, we found that the deletion of the BMP receptor genes Bmpr1b and Bmpr1a results in a loss of all dorsal midline cell types without affecting the specification of cortical and ventral precursors (Fernandes et al., 2007).
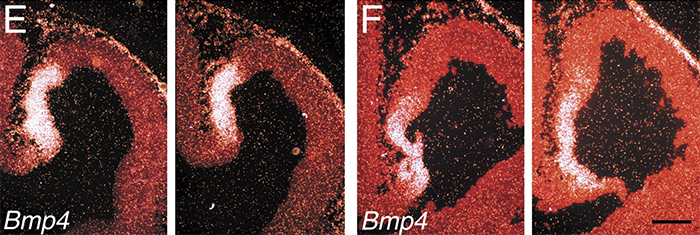
Foxg1-Cre mice were crossed with mice bearing a conditional allele of Bmpr1a (Hébert et al., 2002), which severely compromises BMP signaling at the dorsal midline. The most medial telencephalic derivative, the choroid plexus, fails to differentiate in these mice, as evidenced by the loss of Ttr expression. Cells in this domain do not appear to acquire an alternative fate.
We also examined the role of FGF signaling in telencephalic patterning. Foxg1-Cre mice were crossed to mice that carry a conditional allele of Fgfr1 (Hébert et al., 2003). In these mutants, the olfactory bulbs fail to form normally. This failure occurs despite specification of anterior telencephalic cells, a normal early accumulation of anterior neurons that go on to form connections characteristic of mitral cells, and the presence of radial glia. What differs between the control and Fgfr1-deficient telencephalon is that the normal decrease in cell proliferation at the anterior tip of the brain fails to occur during the initial stages of OB morphogenesis, indicating that Fgfr1 is directly or indirectly required to inhibit anterior cell proliferation and promote OB evagination. In addition to Fgfr1, Fgfr2 and Fgfr3 are also expressed in neural precursor cells. In studies performed primarily in Jean Hébert's labortatory at Albert Einstein, we have deleted all combinations of pairs of Fgfr genes in the telencephalon using a conditional genetic approach in the mouse (Gutin et al, 2006). Although the dorsal telencephalon remains grossly normal in all double mutants, the ventral telencephalon shows distinct phenotypes in two of the these mutants. First, in the Fgfr1;Fgfr3 mutant, differentiated ventro-medial cells that normally express Lhx6 and Lhx7 are lost, despite the presence of their Nkx2.1-expressing precursors, indicating a role for FGF signaling in ventral cell differentiation. Second, in the Fgfr1;Fgfr2 mutant, a more severe phenotype is observed in which ventral precursor cells are lost, leading to a dorsalized telencephalon. although this phenotype mimics that of a telencephalic-specific loss of SHH signaling, Shh and Gli1 are still expressed at detectable levels ventrally. The Fgfr1;Fgfr2 phenotype, unlike the Shh phenotype, is not rescued by loss of the Shh antagonist Gli3, further indicating that FGF signaling acts downstream of Shh and Gli3 to induce ventral telencephalic cell types. Finally, in collaboration with the labs of Gail Martin and John Rubenstein at UCSF, we have found that alterations of FGF8 signaling profoundly disrupt telencephalic patterning, and that it does so in a concentration-dependent manner (Storm et al., 2006).
The division of the forebrain into distinct left and right hemispheres represents a critical step in neural development. Several signaling molecules, including SHH, FGF8, and BMPs, have been implicated in dorsal midline development, and prior work suggests that the organizing centers from which these proteins are secreted mutually regulate one another during development. To explore the role of the ventral organizing center in the formation of two hemispheres, we assessed dorsal midline development in Shh mutant embryos and in wildtype embryos treated with the SHH signaling inhibitor HhAntag (Hayhurst et al., 2008). Collectively our findings demonstrate that SHH signaling plays an important role in maintaining the normal expression patterns of Fgf8 and Bmp4 in the developing forebrain. We further show that FGF8 can induce the expression of Zic2, which is normally expressed at the midline and is required in vivo for hemispheric cleavage, suggesting that FGF signaling may stimulate dorsal midline development by inducing Zic2 expression.
References:
Hayhurst M, Gore BB, Tessier-Lavigne M, McConnell SK (2008) Ongoing Sonic hedgehog signaling is required for dorsal midline formation in the developing forebrain. Devel. Neurobiol., 68:83-100.
Fernandes M, Gutin G, Alcorn H, McConnell SK, Hébert JM (2007) Mutations in the BMP pathway in mice support the existence of two molecular classes of holoprosencephaly. Development 134:3789-3794.
Storm E, Garel S, Borello U, Hébert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JLR (2006) Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development 133:1831-1844.
Gutin G, Fernandes M, Ornitz D, McConnell SK, Hébert JM (2006) FGF signaling generates ventral telencephalic cells independently of SHH. Development 133: 2937-2946.
Hayhurst M, McConnell SK (2003) Mouse models of holoprosencephaly. Curr. Opin. Neurol. 16:135-141.
Hébert JM, Lin M, Partanen J, Rossant J, McConnell SK (2003) FGF signaling through FGFR1 is required for olfactory bulb morphogenesis. Development 130:1101-1111.
Hébert JM, Mishina Y, McConnell SK (2002) BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron 35:1029-1041.
Hébert JM, McConnell SK (2000) Targeted introduction of Cre into the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Devel. Biol. 222:296-306.
Hébert JM, Hayhurst M, Marks ME, Kulessa H, Hogan BLM, McConnell SK (2003) BMP ligands act redundantly to pattern the dorsal telencephalic midline. Genesis 35:214 -219.