Wnt proteins
Most mammalian genomes, including the human genome, harbor 19 Wnt genes, falling into 12 conserved Wnt subfamilies. At least 11 of these subfamilies occur in the genome of a Cnidaria emphasizing the crucial role that Wnt proteins play in organismal patterning throughout the animal kingdom. Even sponges contain a few Wnt genes, while single cell organisms do not, suggesting that during evolution Wnt signaling was instrumental in the origin of multi-cellular animals.
Wnt secretion is complex and requires several dedicated components, including Wntless/Evi and Porcupine.
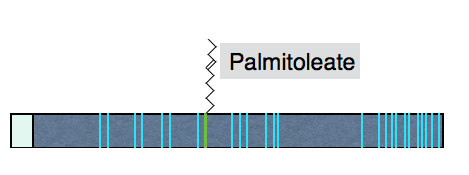
Wnt proteins are approximately 40 kDa in size and contain many conserved cysteines. Wnts are modified by a mono-unsaturated fatty acid (palmitoleic acid), attached to a conserved serine. The lipids on Wnts are required for efficient signaling and may be important for Wnt secretion. The structure of the Xenopus Wnt8 protein as bound to a Frizzled CRD moiety (the Wnt binding domain on the Frizzled receptor reveals two domains on Wnt that interact with the receptor. Interestingly, one of these domains contains the palmitoleic acid lipid which projects into a pocket in the Frizzled CRD, a configuration that reinforces the importance of the lipid for signaling.
The structures of 2 Wnt proteins have been elucidated: Xwnt8 and WntD.