|
Unlike
the Gag proteins, the Env polyprotein precursor is transcribed
from a spliced, subgenomic mRNA which contains no packaging
signals, and is cleaved into the mature SU and TM proteins by
the action of a cellular protease. In most cases the two proteins
are held together by disulfide linkages, as well as by noncovalent
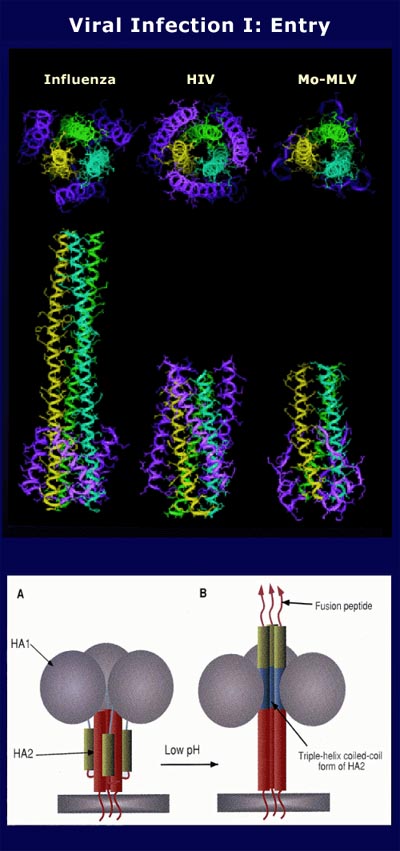
interactions. Although not enzymatically active, Env proteins
have structural features similar to the HA polyprotein of influenza.
The Env heterodimer also forms into a higher order structure,
most probably a trimer or tetramer of dimeric units.
SU
encodes the major neutralizing epitope of the virus and is responsible
for receptor binding at the surface of susceptible cells. SU
proteins are always N-glycosylated, in fact they are unusually
heavily glycosylated, and these glycosylation sites are fairly
well conserved between related viruses. Intramolecular cysteine
bonds are found in completely conserved positions outside of
highly variable regions. Determinants for receptor binding in
SU have been mapped in some cases, however, no rules to explain
receptor choice have been identified thus far.
TM
is usually N-glycosylated, although not in the mammalian Type
C viruses and not heavily. It has a hydrophobic domain which
spans the cell and virus envelope. The outer domain of the protein
has a relatively hydrophobic N-terminus referred to as the fusion
domain thought to contact the cell surface after a conformation
change induced by SU binding to receptor. Whether it too then
binds to a specific cellular receptor is not known, but is an
active area of current investigation. Most TM proteins have
a short cytoplasmic domain, although the primate AIDS lentivirus
TM have a long cytoplasmic domain. Presence or absence of this
tail extension dictates host range and perhaps the stability
of the protein, but otherwise its function is unknown.
SU
proteins are often critical determinants of the pathogenic "virulence"
and disease-type specificity of the virus. This is due to several
more or less known factors: Since SU is responsible for receptor
choice, the host cell-specific expression of the receptor in
part determines the host range of the virus. Secondly, the interaction
between SU and the receptor can affect cellular processes, perhaps
specific to the cell type or stage of differentiation or activation.
Lastly, expression of the protein at the cell surface or internally
can result in both immune targeting as well as unknown effects
on normal cellular processes.
|