|
Research
Cytochrome c oxidase
Nitric Oxide Reductase
Surface Modification
Metal–metal bonds
Public education
Publications
People/Photos
Group Only
Professor
Collman
About (official)
Curriculum Vitae
Department of Chemistry
Stanford University
Useful
links for JPC
Coursework
axess
Away-from-my-mail message
e-journals
|
News
and Views
- A
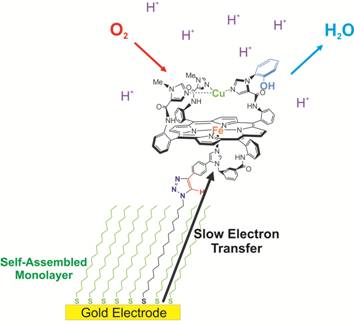
Cytochrome c Oxidase Model Catalyzes the Reduction of Oxygen to Water
Under Rate-Limiting Electron Flux
We studied the selectivity of a functional model of cytochrome c
oxidase's active site that mimics the coordination environment and
relative locations of Fea3, CuB, and Tyr244.
To control electron flux, we covalently attached this model and analogs
lacking copper and phenol onto self-assembled monolayer–coated gold
electrodes. When the electron transfer rate was made rate limiting, both
copper and phenol were required to enhance selective reduction of oxygen
to water. This finding supports the hypothesis that, during steady-state
turnover, the primary role of these redox centers is to rapidly provide
all the electrons needed to reduce oxygen by four electrons, thus
preventing the release of toxic partially reduced oxygen species.: Science, 2007, 315, 5818, 1565-1568.
DOI: 10.1126/science.1135844. Lead authors: Neal Devaraj,
Richard Decreau.
- Collmania 2006
Photos from the 2006
Collman Symposium are available here.
- Interaction of
nitric oxide with a functional model of cytochrome c oxidase. Cytochrome c oxidase (CcO) is
a multimetallic enzyme that carries out the reduction of O2 to H2O and
is essential to respiration, providing the energy that powers all
aerobic organisms by generating heat and forming ATP. The oxygen-binding
heme a3 should be subject to fatal inhibition by chemicals that could
compete with O2 binding. Near the CcO active site is another enzyme, NO
synthase, which produces the gaseous hormone NO. NO can strongly bind to
heme a3, thus inhibiting respiration. However, this disaster does not
occur. Using functional models for the CcO active site, we show how NO
inhibition is avoided; in fact, it is found that NO can protect the
respiratory enzyme from other inhibitors such as cyanide, a classic
poison: Proc.
Natl. Acad. Sci. U. S. A., 2008, 105(29), 9892-9896. Lead
authors: AbhishekDey, Richard Decréau, Ying
Yang
- A Functional
Nitric Oxide Reductase Model. The
first functional heme/non-heme nitric oxide reductase (NOR) model is
presented. The fully reduced diiron compound reacts with two equivalents
of NO leading to the formation of one equivalent of N2O and the
bis-ferric product. NO binds to both heme Fe and non-heme Fe complexes
forming individual ferrous nitrosyl species. The mixed-valence species
with an oxidized heme and a reduced non-heme FeB does not show NO
reduction activity. These results are consistent with a so-called
"trans" mechanism for the reduction of NO by NOR: Proc.
Natl. Acad. Sci. U. S. A., 2008,105(41), 15660-15665.. Lead
authors: Ying Yang, AbhishekDey, Richard Decréau
|