Human
| Immunodeficiency
Home |
Schimke Immuno-Osseous Dysplasia (SIOD)- T cell immunodeficiency
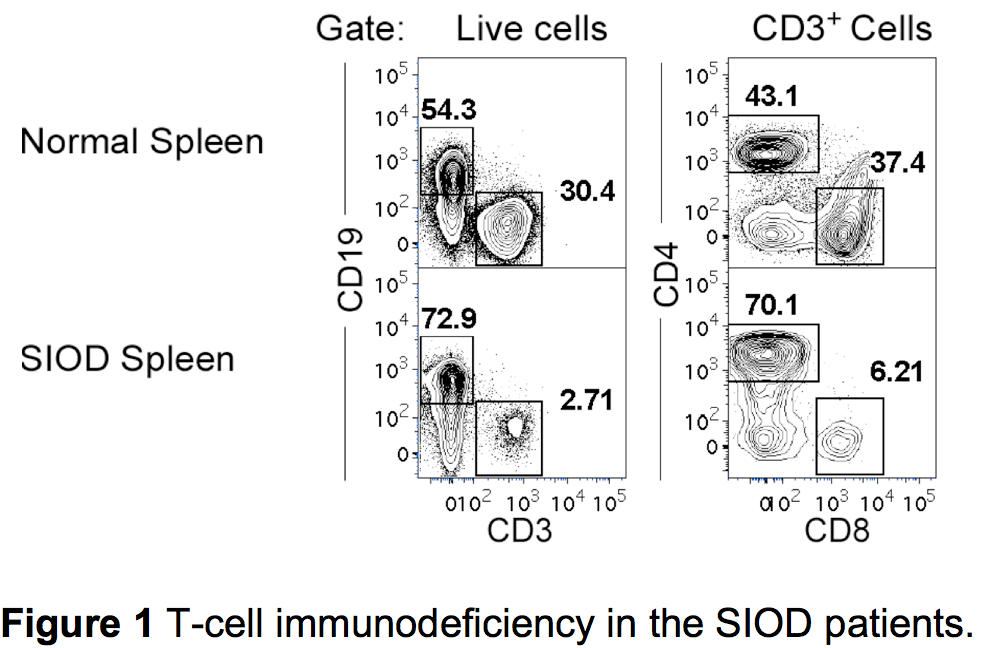
Schimke immuno-osseous dysplasia (SIOD) is an autosomal recessive, incompletely penetrant, childhood disorder associated with skeletal dysplasia, renal dysfunction, and T-cell lymphopenia (Figure 1). The representation of T-cell subsets in SIOD patients is further characterized by a high proportion of memory (CD45RA-CD45RO+) T cells, however, the etiology of T-cell immunodeficiency has not been elucidated. I found that T cells in individuals affected by SIOD express markedly reduced surface levels of IL-7 receptor alpha chain (CD127) as compared to their unaffected sibling or normal individuals. This reduction in IL-7 receptor alpha chain was observed in all T-cell subsets including naïve T cells. This suggests that the origin of the IL-7 receptor alpha chain deficiency is likely intrathymic, rather than occurring after emigration to the periphery. In addition, T cells from SIOD patients proliferate poorly in response to stimulation with IL-7, indicating a loss of functional receptor. From these observations, I propose the lack of functional IL-7 receptor expression in T cells, and possibly their earlier progenitors, may have restricted T-cell development in SIOD patients. Currently, I am trying to elucidate the mechanism for this reduced IL-7 receptor expression.
Development of mouse:human hematopoietic chimera to study human immune system
Understanding normal lymphocyte development and function in human is limited due to inaccessibility of normal lymphoid tissues. Similarly, the understanding the etiology of many human hematopoietic diseases is limited to obtainable peripheral blood cell. Since, hematopoietic stem cells can regenerate entire blood system, xeno-transplantation of these cells are useful to recapitulate normal as well as pathological human hematopoietic development. Currently, I am utilizing this approach to generate mouse:human hematopoietic chimera where human hematopoietic progenitor cells (CD34+ or CD133+) from various sources like fetal liver, cord blood, adult peripheral blood, mobilized peripheral blood are being transplanted into immuno-deficient mice. Currently, I am assessing the ability of these progenitors to reconstitute human immune system in mice. Subsequently, I will seek collaboration to obtain adult human progenitor cells from specific immune disorders to reconstitute their immune system in immune deficient mice. This will allow me to interrogate the steps of normal human lymphoid development as well as etiology of specific immune disorder.
Publications
2015, Clinical Immunology 161 (2): 355
2012, Human Molecular Genetics 21(11): 2572
2010, Blood Nov 2010; 116: 2767 (conference abstract)