Collagen / Hyaluronic Acid Matrices
for Connective Tissue Repair
Eric E Sabelman PhD, Nicole Diep MS, ¹
William Lineaweaver MD ²
¹VA Rehabilitation R&D Center
3801 Miranda Ave., MS-153
Palo Alto, CA 94304
² 
Stanford University
Division of Plastic & Reconstructive Surgery
Medical School, M/C 5560
Stanford, CA 94305
Abstract
Subcutaneous tissue loss from pressure sores or trauma is commonly treated
by reconstructive surgery, using a myocutaneous flap rotated from an adjacent
unaffected site. This is a preliminary report on a semi-synthetic graft
intended to perform the same function as the surgical flap but without donor
site morbidity; it should have the same properties as lost tissue and contain
autologous cells. We are studying composites of Type I bovine or rat collagen
with hyaluronic acid (HyA).
Three physical forms of 1:1 collagen:HyA have been made:
- (1) homogeneous dispersions of HyA particles,
- (2) continuous-strand esterified HyA mats or felts,
- (3) loosely-packed HyA ester beads.
Physical properties were assessed by a sphere indentation test for creep
compliance and a tensile relaxation test using sutured junctions simulating
clinical use. Creep (combined compression and shear) and tensile moduli were
lower but comparable to published values for rabbit mesentery including effects
of pre-loading history and continued creep over long periods (>16 hours).
Early (<2 minute) indentation is influenced by a collagen-rich surface
layer. Cell interaction: Rat cells were added in the form of: neonatal
fibroblasts layered onto the matrix, adult omentum explants inset into the
matrix, and adult fibroblasts and adipocytes distributed throughout the matrix.
Cell survival and attachment were assessed after 7-14 days by vital staining
and post-fixation histology. Survival was poor (<50%) in homogeneous
collagen:HyA, due to residual solvent in the HyA. Cells migrating from explants
tended to envelop individual HyA beads or strands. Surface cells entered the
matrix principally along flaws in the collagen phase. HyA prevented contraction
seen in cell-seeded collagen-only preparations. Matrices of stranded HyA with
cells distributed in the collagen phase have better strength and surgical
handling, and are being pursued further.
Return to Contents
Problem
Pressure sores ("decubitus ulcers") occur in hyposensitive skin of
immobilized individuals, where prolonged compression of tissue between the
supporting surface and a bony prominence impairs capillary blood perfusion in
the region 1 . Sufferers typically include
paralyzed spinal cord injured and stroke patients, postsurgical and casted
fracture patients, and frail institutionalized elderly 2 . It is estimated that every year 7% of the 200,000
spinal cord injured persons in America will develop an ischial pressure sore.
Additionally, 14% of the general population over 70 years of age will develop
pressure sores. Between 3 and 8.8% of sores will require surgery 3 [Figure 1].
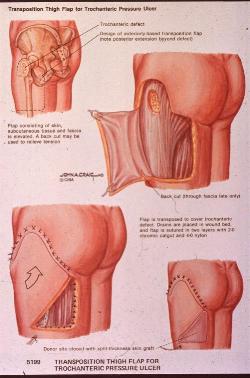 |
| Figure 1 - Pressure sore surgery |
Recurrence rates after surgery vary from 7% in trochanteric ulcers to 11% of
sacral sores 4 ; recurrence is frequently due to
continued stress on deep tissues despite adequate skin coverage. All pressure
sores are difficult to treat; failed surgeries result in some of the longest
hospital stays (>70 days on average) and highest costs.
Conservative dressings: The range of nonsurgical approaches to enhance
healing of open pressure sores has recently broadened. Gauze dressings have
been superceded by a variety of packings (usually based on sodium alginate
5 ) and film barriers for controlling moisture
content of the wound environment while excluding contamination. Collagen
6,7,8
and collagen:HyA 9 have been investigated as
dressings, but without tailored microgeometry to encourage incorporation into
living tissue.
 |
| Two-phase collagen:hyaluronic acid composite
tissues |
Reconstructive surgery using prefabricated flaps: Since restoration and
maintenance of vascular continuity within any flap is essential to its
survival, prefabrication is used to either place a larger vessel within tissue
to be transferred or to prepare a donor site in advance, thus achieving in a
single procedure what would otherwise require multiple operations 10 . Experimentally, synthetic biomaterials have been
combined with mobilization of a vascular pedicle, which accelerates ingrowth of
connective tissue into the synthetic graft 11.
Similar concepts have been explored by Walton and Brown 12 , with vascular pedicles to accelerate
vascularization of Teflon discs, and Mikos and co-workers 13 , who implanted polylactic acid discs into folds in
the rat mesenteric membrane.
Return to Contents
Objectives
We hypothesize that a cell-seeded synthetic matrix could perform some of the
functions of the myocutaneous flap autograft, specifically:
- Restore connective tissue depth and contour better than a skin graft
- Restore mechanical properties for protection against vascular stasis under
load
- Restore viable tissue more rapidly than healing by peripheral ingrowth
The success of the tissue-engineered graft would be enhanced by:
- Rapid revascularization
- Reducing the magnitude of the interfacial barrier between graft and wound
bed, so as to prevent fibrous encapsulation
The immediate goals reported in this paper are to:
- Fabricate tissue matrices having properties approximating subcutaneous
connective tissue
- Examine interaction of these matrices with cells in vitro
- Implant matrices with a vascular pedicle into animals and retrieve after 3,
5, and 8 weeks
Return to Contents
Approach
The graft is constructed of a biopolymer matrix inoculated with fibroblasts
and/or epidermal cells and is either microsurgically reconnected to blood
vessels near the wound, or perfused with a culture medium in lieu of blood
circulation.
The matrix consists of collagen, which provides a substrate for cell
attachment, and hyaluronic acid, which provides bulk, viscous damping and
compression resistance without impeding cell mobility 14 , similar to fat in the intact tissue. Collagen is
an appropriate material for such matrices, since it is a structural protein
with minimal interspecies immunoreactivity and capable of dissolution and
controlled repolymerization 15 .
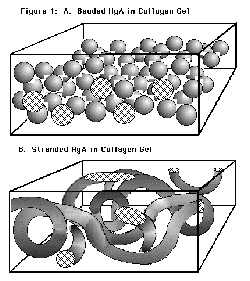
 |
| Figure 2 - HyA:collagen matrix |
|
Three physical forms have been created [Figure 2]:
- homogeneous 1:1 collagen:HyA dispersions of random-geometry HyA particles
- continuous-strand HyA mats or felts
- loosely-packed HyA beads
Note that because HyA is in the form of a discrete micron-scale phase, it
does not interact with collagen at the molecular level as in cartilage 16 , nor is it free to flow under load 17.
|
Return to Contents
Methods 1
The matrices under investigation were mixtures of Type I collagen (from
bovine skin or rat tail tendon) with hyaluronic acid (supplied in 1:9 DMSO
solution). Collagen (1-2% in pH 3.5 acetic acid, neutralized by mixing 1:1
Hanks BSS + stoichiometric NaOH) was added to preformed HyA bead or strand
preparations and allowed to polymerize in situ in 35-100 mm Petri dishes.
Cell-free matrices were cross-linked by UV illumination (254 nm, ¸ 200
mw/cm2) to avoid cross-linking agents such as glutaraldehyde known to have
cytotoxic effects 18 .
| Homogeneous Collagen/HyA - Foams of Type I collagen are produced
by neutralizing the acid collagen solution and homogenizing to disperse
entrapped air and CO2 formed during neutralization into micron-scale bubbles.
The homogenized collagen is mixed at various ratios with 5% HyA in DMSO using
the same method but lower shear forces, then is layered into a Petri dish and
allowed to polymerize or gel in situ for two hours at 37· C. The
microbubbles dissolve and are replaced by liquid over the course of a few days,
leaving a porous fluid-filled microstructure.
Collagen/HyA bead matrix - Droplets or beads of hyaluronic acid are
formed in an immiscible liquid using extrusion nozzles driven by a
low-frequency oscillator. The beads are uniform in diameter (50 µm - 1
mm) and therefore incite minimal foreign-body reaction compared to dispersions
formed by sonication[Figure 2b]. Similar methods are used for
microencapsulation of cells for suspension culture and xenogeneic
transplantation 19 .
|
 |
| Figure 2b- Collagen/HyA bead matrix - |
|
 |
| Figure 3a - -Beaded HyA:collagen |
|
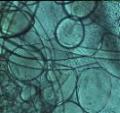
Packing density of the beads [Figure 3a] is such that spaces between them
permit immigration of cells from an inoculum or the periphery of the
wound. |
 |
| Figure 3b - Collagen/HyA felt matrix |
|
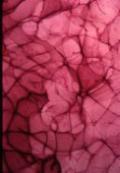
Collagen/HyA felt matrix - Fibers or strands of HyA are made by
extrusion into a bath (e.g.: absolute ethanol) that is miscible with the DMSO
solvent but immiscible with HyA [Figure 3b]. |
 |
| Figure 4a - Stranded HyA:collagen |
|
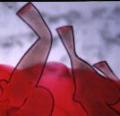
Prolonged soaking (2-7 days) in a similar bath results in condensed HyA
strands that have high tensile strength and resistance to rehydration compared
to bulk HyA [Figure 4a].17. |
 |
| Figure 4b - Stranded HyA:collagen |
|
The soaking bath is removed by filtration and washing with distilled water;
this and mechanical treatment to interlock the strands yields a felt or mat 1-2
mm thick with interstices between strands of 50 µm to several mm [Figure
4b]. |
Return to Contents
Methods 2
Donor Cells - For in vitro testing, tissues were
obtained from neonatal or young adult Fisher rats. Specimens were dissected
using aseptic microsurgical technique into deep and superficial components,
then enzymatic or immunoadhesion techniques were used to separate epidermal
cells, fibroblasts, adipocytes and/or myoblasts. For clinical use, autologous
cells would be obtained from the wound margin or from biopsy of soft tissues
remote from the injury site.
 |
| Figure 5 - Explant culture in wells or dishes |
In Vitro Culture Model - Cells were cultured on experimental
matrices in either static Petri dishes [Figure 5] or in perfused chambers
[Figure 6]. After expanding cell population in culture, cells were suspended in
DMEM + 10% FBS and added at concentrations of 500-2000 cells/mm3 of matrix. In
some static cultures cells were layered onto the matrix in the same dish in
which it was gelled; for replicate cultures, the matrix was cut into 1 cm
squares and distributed among 4-8 wells or dishes.
 |
| Perfusion circuit for in vitro test |
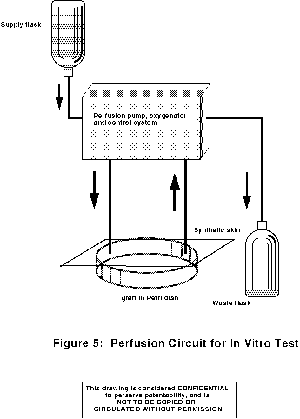
Perfused cultures were in molded silicone chambers with a 25 mm square
cavity; passing through the midline of the 5 mm depth of the cavity were
parallel polysulfone hollow ultrafiltration fibers through which culture medium
(DMEM + 5% FBS) was pumped at 2 ml/min [Figure 6].
 |
| Figure 6 - Perfused silicone chambers |
One or two layers of matrix were placed on each side of the row of hollow
fibers before sealing the chamber between aluminum plates. Cells were
inoculated by injection through the silicone chamber wall. In vitro cell
survival and attachment were assessed after 7-14 days by vital neutral red
staining and post-fixation thin section histology with hematoxylin-eosin
staining. Relative cell population was estimated by comparing area occupied by
cells in and perpendicular to the plane of the matrix layer, with cell-culture
plastic as a control substrate. Matrix geometry was evaluated in compressed
whole mounts stained for collagen with Sirius red F3BA and for HyA with alcian
blue.
Return to Contents
Methods 3
Surgical implantation - Male F344 Fischer rats weighing
approximately 400 grams were anesthetized with intraperitoneal sodium
pentobarbital. All surgical repairs and dissections were performed under a
Zeiss operating microscope with 10X to 25X magnification. A 25 - 30 mm square
patch of skin over the abdominal region was shaved, incised on 3 sides and
elevated HyA strand or bead-containing matrix layers were cut to 20 - 25 mm
square, then laid in place and attached to muscle fascia with 2 or 3 4-0 nylon
sutures. The arteriovenous pedicle was constructed from the saphenous vein
dissected from one leg, looped over the deep matrix layer, and anastomosed to
the femoral artery. Sufficient collateral veins exist so that circulation to
the leg, although initially decreased, is not static. One or more layers of
matrix were laid over the vascular loop before closing the incision with wound
clips.
Rats were sacrificed and the implant region was excised and fixed at 3, 5
and 8 weeks post-surgery. Paraffin sections were cut at 6 to 8 µm and
stained with hematoxylin-eosin to evaluate inflammatory reaction, and with
Masson's or Gomori's trichrome for connective tissue. Because HyA was not
infiltrated with paraffin, displacement and folding of HyA in sections could
not be avoided. Light microscopy was used to visualize: presence and
orientation of blood vessels, location and morphology of cells, orientation and
density of the extracellular matrix, location of specific matrix components
dimensions of voids within the matrix, adhesion to the surrounding tissue, and
extent of biodegradation of the matrix.
Return to Contents
Methods 4
Mechanical Testing - In order to compare synthetic matrices
with host site tissue and with published values for similar materials, physical
properties were assessed by a sphere indentation test for creep compliance, and
a tensile stress relaxation test using sutured junctions simulating the
clinical application.
 |
| Figure 7a - Sphere indentation compression test |
|
Both tests were performed at ambient temperature and humidity.
In the sphere indentation test [Figure 7a], layers of matrix 15 mm in
diameter were stacked on a glass slide to achieve 6-7.5 mm depth. A steel
sphere (1/8 to 7/16 inch diameter stainless ball bearing) was gently placed on
the matrix, and the rate and distance it sank into the matrix were measured on
a video image at 15X magnification. A time-dependent compression modulus, E(t),
was calculated from:
|
|
2 (1-µ2) P
E=------------
_ 2Rh-h2
|
where µ is Poisson's ratio (=0.5), P is the total force generated by the
sphere's weight, R is the sphere's radius, and h is the depth of indentation at
time t. This rudimentary calculation takes into account the change in surface
stress as contact area increases, but assumes internal stresses in the matrix
are roughly equivalent to a flat indentor and ignores effects of finite
thickness 20 . Samples of previously-frozen rat
abdominal fat and skeletal muscle were tested by the same method. |
 |
| Figure 7b - Tensile stress relaxation test |
|
Tensile tests were conducted on 10 x 25 mm
samples typically 2 mm thick [Figure 7b]. Monofilament polypropylene suture
(4-0) was used to make 3 or 4 stitches in each end; the upper end was tied to a
beam attached to one arm of a Cahn microbalance. After taring the balance, the
other end was fixed to a 20 gram weight on an elevating stage. Weights (1-14
gram) were added to the opposite arm and the balance was re-leveled by lowering
the stage a measured distance. Stress relaxation was measured by nulling the
balance at intervals during a 10-180 minute period. Tensile modulus was
calculated assuming a constant cross-section. The same apparatus can be used
for suture pull-out tests, which typically require forces greater than 20
grams.
|
Return to Contents
Results 1
Matrix Geometry - Sections of homogeneous collagen:HyA
matrices showed that HyA was uniformly dispersed in the form of sharp-edged
laminated particles less than 200 µm, typically 20-50 µm, in
diameter.
| Missing figure - fig8.jpg

|
| Figure 8 - HyA strands under tension relative to collagen
gel |
|
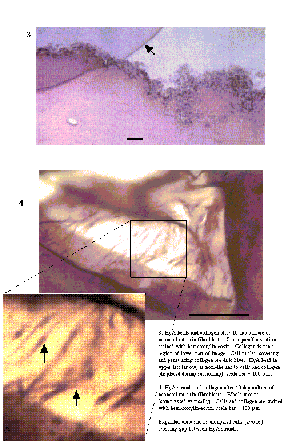
HyA strands were 100-200 µm diameter and in compressed whole
mounts were interlaced and effectively coated with collagen. The stiff HyA
strands were subject to residual bending stress, as seen at cut edges where
they spring out of the surrounding collagen gel [Figure 8]. HyA beads were
0.5-1.0 mm diameter and sparsely distributed, separated by 2 or 3 diameters of
collagen gel.
|
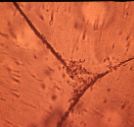 |
| Figure 9 - Cells penetrating defects in collagen
phase |
|
Cell growth - Cells were initially concentrated at the surface
of matrices, and over the first week of culture penetrated into defects in the
collagen phase [Figure 9]. Neither cells nor collagen adhered to HyA beads
other than by mechanical entrapment.
|
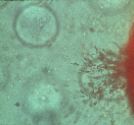 |
| Figure 10 - Cells migrating through collagen phase in
vitro |
|
Populations of cells migrated through the collagen phase without
directly contacting the HyA phase [Figure 10]. Unconstrained pure collagen gels
are known to contract under the influence of cell-generated tensile forces;
this did not occur in HyA-containing matrices. Passage of cells completely
through the matrix was avoided in perfused cultures by turning the chamber over
once a day. Such leakage of cells seemed more prominent in regions with sparse
HyA fibers. While quantitative cell viability tests were not conducted, fewer
cells survived on or in homogeneous collagen:HyA preparations compared to
matrices containing pre-formed HyA or to tissue-culture plastic.
|
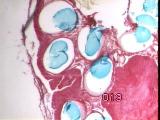 |
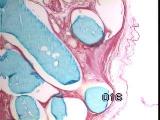 |
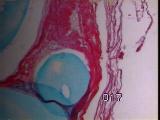 |
|
Figures 11a,b,c - Implant at 3 weeks: neovascularization, collagenolysis
In vivo implantation - Inflammation was minimal, with rapid capillary
outgrowth into the matrix within 3 weeks, accompanied by evidence of
collagenolysis [Figure 11].
|
|
 |
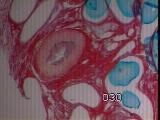 |
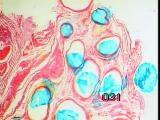 |
|
Figures 12a,b,c - Implant at 5 weeks: acute inflammation subsided
At 5 weeks, acute inflammation had subsided, although chronic inflammatory
cells were present [Figure 12].
|
|
 |
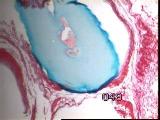 |
 |
|
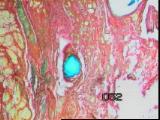
Figures 13a,b,c - Implant at 8 weeks: HyA component largely intact
At 8 weeks, most of the original collagen had been replaced by fibrous
connective tissue, while the HyA component was largely intact [Figure 13]
vascularity was reduced.
|
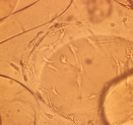 |
| Figure 14 - Cells enveloping individual HyA beads or
strands |
|
As predicted from in vitro cell ingrowth experiments, migrating cells
enveloped individual HyA beads or strands, and did not form a fibrous capsule
around the entire implant [Figure 14].
|
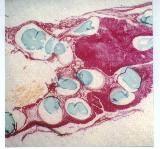 |
| Figure 15a - Vascular pedicle, cross section |
|
The collagen phase was nearly all resorbed by 8 weeks; in contrast,
most HyA beads or strands were intact [Figure 15a]. Foreign body giant cells
were present, but rare, adjacent to HyA surfaces. Individual HyA strands or
beads became encapsulated by a 3 - 4 cell thick layer.
|
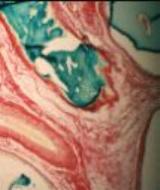 |
| Figure 15b - Vascular pedicle, longitudinal
section |
|
The vascular pedical was evident in both cross [Figure 15a] and
longitudinal section [Figure 15b]. The abdominal site did not provide a wound
margin suitable for testing integration.
|
Return to Contents
Results 2
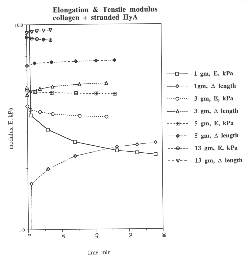
Mechanical Properties -
Return to Contents
Discussion
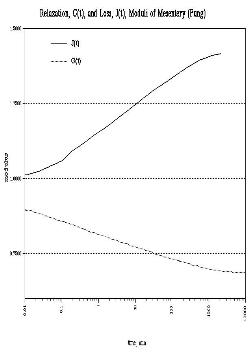 |
| Figure 19 - Creep & relaxation moduli of rabbit
mesentery (Fung [23]) |
|
Material properties of soft tissues tested as controls were
comparable to published values for similar tissues (Fung22,23) gives tensile creep and
relaxation moduli for rabbit mesentery, noting that a known pre-loading history
is essential to derivation of repeatable elastic constants, and that over
extremely long periods (¸103 minutes) soft tissues may exhibit unlimited
strain [Figure 19].
This effect is evident in the approximation of indentation to an inverse
power function with respect to time. In our specimens, the early (<2 minute)
curve is most likely due to the collagen-rich surface of the collagen:HyA
composite. Only after the stress field generated by the sinking sphere reaches
the intermeshed HyA strands does this component alter the apparent modulus.
|
|
|
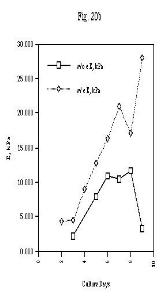
In a study of cell-seeded collagen sponges, Jain, et
al., 24 conducted a tensile test of samples with and without
cells. Stress/strain curves were comparable up to 50% strain [Figure 20].
. Healing of tissue is hypothesized to be accelerated by implantation of a
synthetic tissue having the same chemical and structural properties as the lost
tissue and inoculation of the implant with autologous cells. This concept is
based on two premises: (1) that the human body lacks in maturity geometric and
biochemical cell guidance information present in the embryo, which may be
provided by an artificial extracellular matrix, and (2) that a completely inert
biomaterial lacks the capacity to integrate with intact tissue and respond to
functional demands, provided by a cellular component.
|
The general concept of facilitated regeneration through the use of composite
artificial/cellular tissues has broad applications beyond the current clinical
uses in skin repair and experimental use in peripheral nerve grafts. The
pressure sore is a good model for this concept since it is localized,
accessible without internal surgery and non-critical to immediate survival. The
in vitro test is in fact a worst-case approximation of an avascular
injury site, and if cell growth occurs as desired in vitro, growth
should be as good or better in vivo.
Return to Contents
Acknowledgements
Histology performed by Min Hu, MD PhD; Graphics support by Betty Troy.
Funding by VA Rehabilitation R&D pilot projects B92-476AP, B1389-AP;
in-kind support by Stanford Div. of Plastic & Reconstructive Surgery
Return to Contents
|
References
|
1. |
Vidal, J., Sarrias, M., "An analysis of the diverse factors concerned with
the development of pressure sores in spinal cord injured patients",
Paraplegia, 29: 261-267, 1991. |
2. |
Staas, W.E. Jr., LaMantia, J.G., "Decubitus ulcers and rehabilitation
medicine", Int J Dermatol, 21: 437, 1982. |
3. |
Allman, R.M., Goode, P.S., Patrick, M.M., Burst, N., Bartolucci, A.A. Pressure
ulcer risk factors among hospitalized patients with activity limitation.
JAMA, 273(11): 865-870, 1995. |
4. |
Rogers, J., Wilson, L.F., "Preventing recurrent tissue breakdowns after
'pressure sore' closures", Plast Reconstr Surg, 56: 419,
1975. |
5. |
Chapuis, A., Dollfus, P., "The use of a calcium alginate dressing in the
management of decubitus ulcers in patients with spinal cord lesions",
Paraplegia, 28: 269-271, 1990. |
6. |
Marzin, L, Rouveix, B., "An evaluation of collagen gel in chronic leg
ulcers", Schweiz Rundschau Med (PRAXIS), 71: 1373-1378,
1982. |
7. |
Leipziger, L., Glushko, V., Nichols, J., DiBernardo, B., Shafaie, F., Noble,
J., ALvarez, O.M., "Dermal wound repair: The role of collagen matrix
implants and synthetic polymer dressings", J Am Acad Dermatol,
12: 409-19, 1985. |
8. |
Doillon, C.J., Whyne, C.F., Brandwein, S., Silver, F.H., "Collagen based
wound dressing: Control of the pore structure and morphology", J Biomed
Mater Res, 20: 1219-28, 1986. |
9. |
Doillon, C.J., Silver, F.H., "Collagen based wound dressing: Effects of
hyaluronic acid and fibronectin on wound healing", Biomaterials,
7: 3-8, 1986. |
10. |
Khouri, R.F., Upton, J., Shaw, W.W., " Principles of flap
prefabrication", Clinics in Plastic Surgery, 19(4): 763-772,
1992. |
11. |
Lineaweaver, W., de la Pena, J.A., Briones, R., Yim, K., Newlin, L., Buncke,
H., "Muscle transplantation in the rat" Studies of the latissimus
dorsi, serratus anterior, combined latissimus serratus, and gracilis",
Proc 3rdVienna Muscle Symp <03, 1990>, G. Freilinger & M.
Deutlinger, eds., (Blackwell, Vienna), 3: 9-12, 1992. |
12. |
Walton, R.L., Brown, R.E., "Tissue engineering of biomaterials for
composite reconstruction: an experimental model", Ann Plast Surg,
30: 105-110, 1993. |
13. |
Mikos, A.G., Sarakinos, G., Lyman, M.D., Ingber, D.E., Vacanti, J.P., Langer,
R., "Prevascular-ization of porous biodegradable polymers",
Biotechnology Bioengineering, 42: 716-723, 1993. |
14. |
Larsen, N.E., Pollak, C.T., Reiner, K., Leshchiner, E., Balazs, E.A.,
"Hylan gel biomaterial: dermal and immunologic compatibility", J
Biomed Mater Res, 27: 1129-34, 1993. |
15. |
Sabelman, E.E.: "Biology, biotechnology and biocompatibility of
collagen", chapter 3 in: Biocompatibility of Tissue Analogs, v.
I, D.F.Williams, ed. (CRC Press, Boca Raton, FL), pp. 27-66, 1985. |
16. |
Scott, J.E., "Proteoglycan-fibrillar collagen interactions",
Biochem J, 252: 313-323, 1988. |
17. |
Gibbs, D.A., Merrill, E.W., Smith, K.A., Balazs, E.A, Rheology of hyaluronic
acid. Biopolymers, 6: 777-794, 1968. |
18. |
van Luyn, M.J., van Wachem, P.B., Damink, L.O., Dijkstra, P.J., Feijen, J.,
Nieuwenhuis, P., "Relations between in vitro cytotoxicity and crosslinked
dermal sheep collagens", J Biomed Mater Res, 26(8):
1091-1110, 1992. |
19. |
Wolters, G.H.J., Fritschy, W.M., Gerrits, D., Schilfgaarde, R. van., "A
versatile alginate droplet generator applicable for microencapsulation of
pancreatic islets", J Applied Biomater, 3: 281-86,
1992. |
20. |
Timoshenko, S., Goodier, J.N. Theory of Elasticity, 2nd ed. (New York:
McGraw-Hill), pp. 366-377, 1951. |
21 |
Guidry, C., Grinnel, F., "Contraction of hydrated collagen gels by
fibroblasts: Evidence for two mechanisms by which collagen fibrils are
stabilized", Collagen Rel Res, 6: 515-529, 1986. |
22. |
Duck, F.A., Physical Properties of Tissue (London: Academic Press), pp.
151-160, 1992. |
23 |
Fung, Y.C., Bio-viscoelastic solids, ch. 7 in: Biomechanics: Mechanical
Properties of Living Tissues (New York: Springer-Verlag), pp. 106-260.
1981. |
24 |
Jain, M.K., Chernomorsky, A., Silver, F.H., Berg, R.A., Material properties of
living soft tissue composites. J Biomed Materials Res, 22(A3):
311-326, 1988. |
Return to Contents
|