THE USE OF A ROBOTIC DEVICE FOR POST-STROKE MOVEMENT THERAPY
Peter S. Lum, Ph.D., Charles G. Burgar, M.D., H.F. Machiel Van der Loos,
Ph.D. Proceedings of the International Conference on Rehabilitation Robotics.
AbstractIn post-stroke upper-extremity movement therapy, a therapist uses passive and active assisted strategies to exercise the limb and assess the patient's recovery. The MIME project has developed a robot for patient-initiated, therapist-supervised movement therapy. This paper presents preliminary results to show that MIME can quantify therapy sessions and can show correlation with accepted clinical measures of functional outcomes.
IntroductionTo evaluate the feasibility and utility of mechanically-assisted post-stroke movement therapy, we have developed a prototype device called MIME (Mirror-Image Motion Enabler; see Fig. 1) that implements two commonly used therapeutic techniques: passive and active assisted movements. Normally, these techniques are applied by a therapist who moves the paretic limb as the patient either remains passive, or actively attempts to contribute to the movement.
Prior to performing clinical trials, we are currently investigating whether improved performance in active-assisted movements (as measured by the interaction forces/torques during robotic assistance) correlates with improved functional recovery of the paretic limb (as measured by an upper extremity Fugl-Meyer examination) [1,2]. A strong correlation must be established between the metrics derived from our measurements and functional status, as well as between exercise task performance and functional recovery.
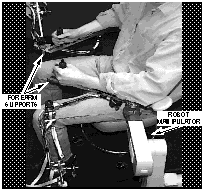
Fig 1. The MIME System.
BackgroundThere is a growing interest in therapeutic applications of robots. The field was recently reviewed in [3]. In one of the earliest initiatives, it was suggested by Khalili and Zomlefer [4] that a two joint robot system could be used for continuous passive motion and could be programmed to the particular needs of the patient. A device with similar clinical, stroke-rehabilitation objectives as MIME, although without MIME's self-initiated aspect of bilateral control, is MIT-MANUS [5]. All of the technology-assisted therapies focus on the issue of individualization of therapies, quantification of outcomes, and the possibility of an increased regimen of therapist-supervised exercise than currently practiced.
MethodsThe MIME system uses two commercial mobile arm supports, which limit arm movement to the horizontal plane, and a 6-DoF robot arm (Stäubli PUMA-260), which applies forces and torques to the paretic forearm through the arm support (Fig. 1.). The choice of horizontal movements allows the robot arm to be relatively small for safety reasons, while still allowing us to address coordinated shoulder and elbow tasks. Optical encoders on the joints of the mobile arm supports measure the position and orientation of the forearms, and a 6-axis force/torque transducer (Assurance Technology, FT3491) measures the forces/torques applied to the paretic limb. Movements can be controlled with pre-programmed forearm position and orientation trajectories, or by a position feedback control system which slaves the robot trajectories to the movements of the contralateral (normal) limb.
Test subjects (n=7) were male, between the ages of 47 and 71, and between 1 and 45 months post-stroke. They were screened for left hemiparesis and the ability to follow simple instructions; all signed the informed consent form to participate in this study. All subjects had a diagnosis of cerebrovascular accident based upon the results of a head CT or MRI scan. To test the repeatability of our measurements, each of the stroke subjects was tested twice within the same week. In addition to the seven post-stroke test subjects, three age-matched able-bodied male subjects participated as normals.
Each test session began with an upper extremity Fugl-Meyer (FM) examination and an Ashworth test of spasticity [1]. Subjects sat in a modified wheelchair and placed their forearms in the arm supports. A chest strap limited torso movement and loss of posture. Straps also secured the forearms to the arm supports. Six different point-to-point reaching movements were tested (Fig. 2). These movements required six different upper limb muscle coordinations, thereby collectively representing overall upper limb function.
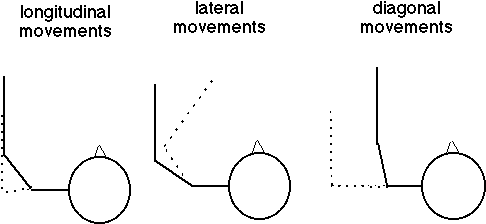
Fig. 2.Top view of the 6 movement types. Movements 1a,2a,3a start from the dashed positions and end at the solid arm positions. Movements 1b,2b,3b are in the reverse directions. In all movements, the hand moves in a straight line 15 cm.
The forearm trajectories of unassisted, 2.5 second duration movements were measured from the three normals. These trajectories were ensemble averaged across subjects to yield six `normal' trajectories, which were programmed into the robot and used in all of the assisted movements. For each movement type, the subject was first instructed to remain passive as the robot moved the limb in the programmed trajectory (5 trials). Next, the subject was instructed to voluntarily contribute to movement by pushing the robot with approximately one pound of force. After each of the 10 trials, the subject was shown his average force level in the direction of movement and was encouraged to increase or decrease his effort accordingly.
Position data from both arm supports and 6-axis data from the paretic-side force/torque sensor were collected at a 100 Hz rate for analysis.
ResultsA quasi-static analysis was used to calculate the forces/torques voluntarily generated (Fv) by the paretic limb during the active trials. At each time point (i): Fv(i)=Fm(i) - Fp(i) For each movement type, Fp(i)=passive profiles ensemble averaged over the 5 trials to estimate the involuntary level at each point during the movement. Fm(i)=the measured forces/torques of each active trial.
The force directional error Øf was calculated as the angle between average force vectors Fv generated by the left and right arms, summed over all time points (i) and then averaged across movement types.
The Fv profiles were also used to calculate the work done by the arm on the robot during each trial (Wa). The work efficiency (h) is defined as: h=Wa / Wp where Wp, the potential work, is defined as the work that would have been produced during a trial if at each instant, the force magnitude were directed precisely in the movement direction, and the torque magnitude was oriented precisely in the direction of rotation.
This averaging of Øf and h is necessary since the FM score reflects overall limb ability. As seen in Fig. 3, the more impaired subjects produced higher force values Fv than less impaired subjects, but these forces were often misdirected relative to the direction of movement. There was a negative correlation between the Fv during a trial and the FM score (p<0.02), with the most impaired subjects (FM scores 14-17) producing forces of approximately 10N, while normals had forces of 6N. However, the force directional error Øf of the paretic limb also was negatively correlated with the FM score (p<0.01), with the Øf of the most impaired subjects approximately 70deg., while for normals, Øf was less than 30deg.. Subjects showed a positive correlation between the work efficiency (h) of the paretic limb and the FM score (p<0.02).
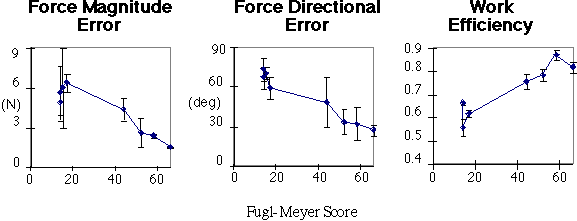
Fig. 3.Results. Graphs show correlations between FM scores and movement metrics (see text).
DiscussionSince the FM score is an overall measure of arm function, the 100 Hz sampled data needed to be averaged over time, trials, and motions to establish a correlation. The use of more fine-grained, individualized metrics, however, may allow us to measure motion artifacts (e.g., spasms) and individual motion patterns to tailor therapies for the individual's own state of recovery.
ConclusionBased on these preliminary results, we will conduct clinical trials to test the therapeutic efficacy of robot assisted limb manipulation. We also plan to use EMG measurements along with the joint torques calculated from a segmental model of the upper limb to describe the impairment in terms of specific muscle force abnormalities.
References1. Katz RT, Rovai GP, Brait C, Rymer WZ. Objective quantification of spastic hypertonia: correlation with clinical findings. Arch Phys Med Rehabil 1992; 73:339-347. 2. Stineman MG, Granger CV. Epidemiology of stroke-related disability and rehabilitation outcome. Phys Med Rehabil Clin North Am 1991; 2(3):457-471. 3. Erlandson RF. Applications of robotic/mechatronic systems in special education, rehabilitation therapy, and vocational training: A paradigm shift. IEEE Trans Rehab Eng 1995; 3(1):22-34. 4. Khalili D, Zomlefer M. An intelligent robotic system for rehabilitation of joints and estimation of body segment parameters. IEEE Trans Biomedical Engineering 1988; 35(2):138-146. 5. Hogan N, Krebs HI, Charnnarong J, Srikrishna P, Sharon A. MIT-MANUS: a workstation for manual therapy and training II. Proc Teleman Tech - SPIE: Intl Soc Optical Eng, 1833, Nov. 1992.
AcknowledgmentsThis research was performed under a grant by the Department of Veterans Affairs Rehabilitation Research Service. We wish to thank Jim Anderson for his help in the design and machining the MIME prototype, to therapists Deborah Kenney and Kim Ho for their clinical assistance, and to the test subjects who gave so willingly of their time to this research.
AddressPeter S. Lum, Ph.D. |