1996 Project Reports | Home |
Contents | Previous |
Next |
Influence of Collar Geometry in femoral components
Jay A. Mandell, MS; Gary S. Beaupré, PhD; Stuart B. Goodman, MD,
Ph.D; David J. Schurman, MD; Dennis R. Carter, PhD
Joint degeneration due to arthritis or trauma adversely affects the quality
of life of many older Americans. Over 250,000 patients are treated yearly with
artificial joint replacements. While these operations have been very successful
in restoring pain-free function, complications can occur, including prosthesis
loosening, infection, inflammation, and bone or prosthesis fracture. The design
of the components for joint replacement surgery can greatly influence the
eventual success or failure of the procedure. At the Rehab R&D Center, we
apply our engineering expertise to investigate how various aspects of component
designs can be improved.
Many current joint replacement components are implanted without the use of
bone cement. Porous surface treatments allow bone tissue to grow into the
prostheses in order to provide stable long-term fixation. One area of concern
for these joint replacements is relative motion (micromotion) between the
prosthesis and the bone in the immediate post-operative period. If too much
micromotion is produced when the patient uses the joint, the bone will not grow
into the prosthesis. Instead of stable fixation, a fibrous (scar-like) tissue
may develop which can lead to pain and eventual failure of the procedure. A
second area of concern is long-term bone adaptation around the prostheses. When
a natural joint is replaced with artificial components, the way the remaining
bone supports the loads generated during daily activities is changed. Bone
hypertrophies with increased loading and atrophies under decreased loading.
Joint replacements generally result in reduced loading of the bone adjacent to
the joint. This can lead to excessive loss of bony support for the prosthesis
and subsequent prosthesis loosening (Fig. 1).
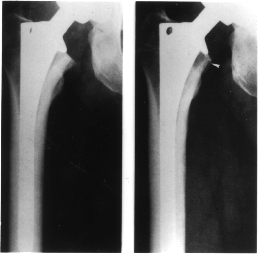 Figure 1. X-rays
of a femoral prosthesis. Compare the bone a) immediately after surgery and b)
after the prosthesis has induced bone loss.
Among the techniques we use to study joint replacements is computer
simulation of bones and artificial components. The most common joint
replacement surgery is total hip replacement, which involves both sides of the
ball and socket joint at the hip. In a recent study, we used computer modeling
to investigate effect of collar geometry on micromotion and bone loss in the
components implanted in the femur (thigh bone).
|
We compared micromotion and load transfer among four prostheses: flat
collared, 30 degree and 60 degree conical-collared, and tapered (Fig. 2).
According to our definition of collar angle, the flat collar and taper are
equivalent to 0 degree and 80 degree conical collars, respectively. To isolate
the effects of collar geometry, the implant models were straight-stemmed and
cylindrical, and collar angle was the only design variable. Our goal was to
quantify the relationship between collar angle, micromotion, and the details of
load transfer in the early post-operative period.
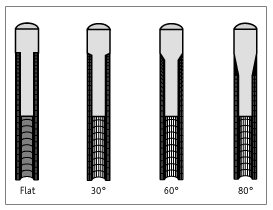 Figure 2.
Computer models of femoral component collar geometries.
The flat (0 degree) collar used direct axial compression to transfer loads
from the joint to the adjacent bone. In contrast, the 30 degree, 60 degree, and
80 degree collars transferred loads by "wedging" into the bone.
Micromotion was lowest in the flat collar model, due to the flat support
provided by the bone, and increased with collar angle, as increased wedging was
accompanied by increased sinking into the bone.
|
To predict changes in bone density around the component, we applied a
mathematical theory for bone adaptation developed by our research group. In
simulating the early post-operative period, we considered a fully loose
(unbonded) prosthesis with no bone ingrowth (Fig. 3). We found that the most
extensive bone loss was predicted for the flat-collared model, and that bone
loss could be expected to decrease with increasing collar angle. Virtually no
bone loss was anticipated in the 60 degree and 80 degree models.
 Figure 3.
Results of the bone adaptation simulation in the bone surrounding the implant.
The initial bone density was assumed to be 1.70 g/cc.
The results suggest that femoral loading can be maintained with
conical-collared or tapered prostheses, but that increased loading is
accompanied by increased micromotion, which may prevent stable long-term bony
fixation. Recent work includes efforts to predict long-term bone adaptation
around these prostheses in the presence of bony ingrowth, and to determine the
effect of the extent of porous coating on bone maintenance.
|
Republished from the 1996 Rehabilitation R&D Center Progress Report. For
current information about this project, contact:
Jay A. Mandell



