Therapeutic vaccines against HPV-induced cervical
carcinoma: Immunogenicity and efficacy of HPV16 and 18 E6 and
E7 proteins as tumor-associated antigens
Abstract
Cervical cancer causes 15% of all cancer deaths worldwide, with
advanced disease carrying a poor prognosis with existing therapies.
HPV has been well established in the etiology of cervical carcinoma,
and in particular the E6 and E7 proteins of HPV types 16 and 18
are the most consistently expressed in tumor cells. The rational
design of therapeutic vaccines to control the growth of HPV-induced
tumors has thus focused on the use of E6 and E7 proteins, or peptides
thereof, as vaccine antigens. A putative CTL epitope of HPV16
E7, peptides 49-57, was identified and shown in various studies
to induce a specific lytic CTL response and to protect against
challenge with HPV16-transformed tumor cells in mice in vivo.
Other vaccine preparations of E6 and E7, containing other peptides
or the entire gene sequences, unaltered or altered, have also
been shown to elicit specific antibody, proliferative, and CTL
responses in mice and in vitro. Peptide immunogenicity was enhanced
in subsequent studies through the use of adjuvants and carriers
including recombinant vaccinia virus (rVV), dendritic cells (DCs),
lysosome-associated membrane protein (LAMP-1), Immunostimulatory
Carrier (ISCAR), and the adjuvant MF59. Several human vaccine
trials are currently underway, but to date, data has only been
published from a small phase I/II trial using an E6/E7-expressing
rVV vector. HPV-specific antibody responses were detected in 3
of 8 late stage cervical cancer patients, and only one patient
demonstrated a HPV-specific CTL response; this study was severely
limited by the requirement of immunocompetence. Most studies have
only looked at one HLA haplotype, and given the HLA polymorphisms
found in the human population, vaccine peptides identified in
this manner may be too restricted in their HLA type and antigen
specificity. Another drawback of using highly specific peptides
is that a peptide that is slightly wrong for an HLA molecule,
either due to mutation or mismatch, could result in T cell anergy.
Most of the research in this area has focused on HPV16 E7, leaving
many potential vaccine peptides and HPV types unexplored. Overall,
there is a need for further elucidation of the immune response
against HPV-induced cancer, and for research in this area to proceed
in a more coordinated and comprehensive fashion.
Introduction
Cervical carcinoma accounts for 15% of all cancer deaths around the world. It is the second leading cause of cancer deaths in women worldwide, with over 500,000 new cases reported each year in developing countries and an incidence of 10-20 per 100,000 women in industrialized nations (14). The role of HPV in the etiology of cervical cancer has been well established. HPV DNA, most commonly types 16 and 18, has been found in over 90% of cervical carcinomas, and a direct association between the prevalence of HPV and the severity of the lesion has been noted (23). It is clear that other cofactors are involved in viral oncogenesis, as less than 1% of women with clinically detectable HPV infection progress to full-blown invasive disease (22). Nevertheless, HPV E6 and E7 proteins are consistently expressed in tumor cells, and their expression is necessary and sufficient for cell transformation and maintenance of malignancy.
Even in the most optimal cases of early detection of low-risk, early stage cervical carcinoma, recurrent disease occurs in 15% of patients, and treatment of recurrent disease is largely unsuccessful (23). The development of more effective strategies for prevention and treatment of cervical cancer is therefore desirable. Immunotherapeutic approaches to treatment are a logical and attractive choice because of cervical cancer's association to viral infection. The HPV E6 and E7 proteins presented in this manner have thus been a primary target for the rational design of prophylactic vaccines to prevent HPV-induced tumor growth as well as therapeutic vaccines to control the growth of already-established tumors.
While various immunotherapeutic strategies against HPV-positive
cervical cancer are currently being pursued, this paper is limited
to those involving the E6 and E7 early proteins (or peptides thereof)
of HPV types 16 and 18. First, some background on the immune response
against HPV infection, mechanisms of immune evasion, and immunotherapy
is presented, with the discussion limited to HPV 16 and 18. Next,
there is a brief overview of the studies to be reviewed and some
common methodologies, followed by in-depth review of research
published in the past 3-4 years. Comments regarding specific studies
are included with the reviews, and general comments on the field
of HPV peptide vaccines for cervical cancer are made at the conclusion
of the paper.
The immune response against HPV
In the past, the study of papillomaviruses has been hampered by
difficulties in applying conventional in vitro culture
methods. HPV only replicates in specific differentiation stages
of epithelia (20), and much remains unclear about the immune response
to HPV infection. Recent advances in molecular biological techniques
have allowed studies to be conducted using recombinant DNA expression
vectors and have also led to the development of synthetic virus-like
particles (VLPs), opening the door for progress in immunologic
studies of HPV.
The antibody response to HPV infection may be involved in virus neutralization and clearance of infected cells via antibody-dependent cellular cytotoxicity (20). Serological studies, however, are complicated by the existence of over 80 HPV types; HPV16 alone exists as several different subtypes which appear to be serologically cross-reactive (7). Some researchers report that antibodies to HPV16 E6 and E7 proteins are found more frequently in patients with invasive cervical cancer and is asso, while others report an inconsistent antibody response among patients with invasive HPV16+ cervical cancer (6,7). Overall, there is agreement that an increased prevalence of E6 and E7 antibodies is seen in carcinoma patients versus controls, but that the incidence of seropositivity does not correspond to likely or documented HPV exposure or the disease state (20).
While elucidation of the humoral immune response to HPV infection will be helpful to the general understanding of the natural course of infection, the cell mediated branch of the immune response is thought to play a greater role than humoral immunity in mediating an effective response against HPV. The focus of researchers has thus been on the cellular response (or markers thereof) to the presentation of HPV antigens, as will become evident in the studies reviewed in this paper.
Several lines of evidence, both epidemiological and from animal models, support the idea that, as is the case with other viral infections, the ability of the cell-mediated immune response to eliminate HPV-infected cells is a crucial determinant of disease outcome. In general, warts and HPV-associated malignancies are observed more commonly in populations with depressed cellular immunity and intact antibody responses, including transplant patients on immunosuppressive therapy and HIV-positive patients (6,23). Regressing flat skin warts show histological evidence of mononuclear cell infiltration, suggesting that cell-mediated immune responses were initiated (6).
The ability of T cells to recognize HPV 16 E6 and E7 has been
clearly demonstrated in mice, and the shift has been to identifying
the naturally processed CTL epitopes and corresponding immune
responses to them in humans. Some of the problems that have been
encountered in studying CMI involve: the nature of the assays
and the HPV antigens available for in vitro stimulation
and as targets, the availability of sufficient and relevant patient
lymphocytes, lack of knowledge about HPV exposure and type, and
the MHC restriction of specific T cell responses (20).
Immune Evasion and Immunotherapy
Tumors often elicit no demonstrable immune response at all, possibly due to cancer-related immunosuppression or the production of paracrine factors promoting tumor growth and tumor cell angiogenesis (13,20). Compromised immunogenicity may also be caused by downregulation of HLA class I molecules, supported by findings of a correlation between reduced HLA-A2 expression and disseminated disease (23). Furthermore, although normal squamous cervical epithelium is negative for HLA class II antigen, over 80% of cervical squamous cell carcinomas express it; the functional significance of this finding is not yet understood. Tumor cells may also lack critical costimulatory molecules such as CD28 and CD80, leading to the induction of tolerance, as another possible mechanism of immune surveillance evasion.
The aim of immunotherapeutic strategies against cancer is therefore
to successfully present tumor-associated antigens to the patient's
immune system via a vaccine, thus inducing an effective immune
response. Immunotherapy is not intended to stand alone, but to
be used in conjunction with conventional surgical and radiotherapy
treatments for cancer. The use of HPV16 and 18 E6 and E7 proteins
makes sense theoretically because E6 and E7 continue to be expressed
in cervical tumors and are required to maintain malignancy, and
their use has been proven successful, at least preliminarily,
in animals.
Overview of studies
Development of a peptide-based vaccine for cervical cancer requires the identification of an appropriate vaccine antigen. Whole sequences of HPV 16 and 18 E6 and E7 have been used, but peptides have the advantage of potentially being able to induce specific CTL responses to a number of subdominant epitopes, decreasing the chance of occurrence of CTL escape variants. With whole tumor cells as the vaccine antigen, the target peptides must be immunodominant CTL epitopes in order to elicit an immune response. Effort was thus focused on identifying immunogenic peptides of E6 and E7, and once potential epitopes were identified, researchers used them to immunize mice and measure the immune responses they elicited, primarily assaying for helper T and cytotoxic T cell activity.
In the studies reviewed, HPV-specific cytotoxic T cell activity is measured by in vitro culture of spleen or lymph node cells from immunized mice with 51Cr- or Eu3+-labelled target cells transfected with the putative epitopes. Specific cell lysis is then measured in terms of release of the marker at varying ratios of effector:target cells. Specific T helper cell proliferative responses are measured by 3H-thymidine incorporation or IL-2 production by cell lines stimulated with the putative epitope(s) in vitro.
Additionally, some authors quantify the antibody response to epitopes using standard ELISA or immunoblot methods. Perhaps the animal experiments which yield the most valuable data are those examining tumor protection and clearance of established tumors in vivo following tumor cell injection. Mice are first immunized and then challenged with tumor cells in the tumor protection assays, or are injected with tumor cells which lead to established tumors and subsequently immunized to evaluate the therapeutic potential of such vaccines.
The use of different adjuvants, such as incomplete Freund's adjuvant (IFA) and MF59, and delivery systems, such as recombinant vaccinia virus (rVV), dendritic cells (DC), Immunostimulatory Carrier (ISCAR), and lysosome-associated membrane protein (LAMP-1) in conjunction with different peptide sequences has been explored. Table 1 shows the HPV types, the peptides and specific peptide sequences, and adjvuants and/or carriers that have been studied in the research to be reviewed in this paper.
author (reference) | HPV type
------------ 16 18 | peptide
------------ E6 E7 |
sequence(s) tested |
adjuvant/ carrier |
| Ressing (17,18) | X | X | 11-20, 86-93 | none |
| Feltkamp (4,5) | X | X | 44-62, 49-57 | none |
| Ossevoort (15) | X | X | 49-57 | DCs, IFA |
| Tindle (21) | X | X | 44-48, 48-54, 49-57 | ISCAR |
| Gao (8) | X | X | entire intron-free sequence | rVV |
| Boursnell (3) | X X | X X | mutated E7 Rb-binding site | rVV |
| Zhu (25) | X | X | 1-52, 48-98 | rVV, MF59 |
| Lin (12) | X | X | entire E7 | rVV, LAMP-1 |
Table 1: Comparison of HPV type, peptides, and adjuvants and/or carriers used in reviewed studies.
Identification of putative CTL epitopes
Feltkamp et al. tested a set of 240 overlapping peptides derived from HPV16 E6 and E7 and defined a CTL epitope based on MHC class I-peptide-binding studies (4). At a relatively low concentration, three peptides bound to Db, one to Kb, and one to both MHC class I molecules; these five peptides were considered candidates for use in a vaccine.
Similar binding studies were conducted by Ressing et al using HPV16 E7-derived peptides (17). Nine HLA-A*201-binding peptides were used to immunize HLA-A*201Kb mice to evaluate the immunogenicity of the peptides; 4/9 induced strong CTL responses. In vitro assays of response induction using PBMCs from healthy HLA-A*201+ donors indicated that at least three of these peptides (sequences 11-20, 82-90, and 86-93) were immunogenic in human beings, and CTLs raised against these three peptides were capable of lysing HLA-A*201+, HPV16+ target cells.
Two of these putative HPV 16 E7 CTL epitopes (sequences 11-20 and 86-93) were then tested for their ability to elicit specific CTL responses in PBMCs from both healthy individuals and patients with HPV16+ CIN or cervical cancer, all of whom were HLA-A*201+ (18). None of the samples responded to E7 86-93. 2/11 CIN patients, 2/11 cancer patients, and 1/10 healthy donors showed a CTL response specific to E7 11-20. The authors suggest that this lack of immunogenicity may have been due to poor HLA class I expression on HPV-infected cells, related to immunodominance, or poor costimulation and cytokine assistance.
Based on previous data identifying HPV16 E7 peptide 44-62 as capable of inducing a strong proliferative response, 49-57 as a putative CTL epitope, and 48-54 as a Th epitope, Feltkamp et al used the 44-62 peptide (containing the putative CTL and Th epitopes) in immunization studies in mice (4). Five immunocompetent mice were immunized with E7 44-62 in IFA and boosted with the same vaccine after 2 weeks, and 5 control mice received IFA alone and were also boosted after 2 weeks. Two weeks after that, all mice were challenged with tumor cells. Tumor development was prevented in 12/13 immunized mice. Further studies conducted similarly showed that the putative CTL epitope (peptide 49-57) alone was sufficient for tumor protection in 19/21 mice. CTL assays indicated that the mechanism of tumor protection provided by E7 49-57 was mediated by CTLs recognizing peptide-loaded target cells.
In an extension of their previous work, Feltkamp et al. demonstrated that HPV16-transformed cells express another CTL epitope besides E7 49-57 which appears to be immunodominant (5). However, CTL raised against the subdominant epitope 49-57 were able to eradicate tumors in 6/6 mice within 3 weeks of administration with IL-2. Tumor growth progressed in control mice treated with IL-2 alone or a different CTL clone plus IL-2.
Comments. These studies represent a good start in identifying CTL epitopes of HPV16, but they are intensively focused on just one very specific possibility for therapeutic immunization for HPV-linked cervical cancer. Other HPV types and other epitopes could also be effective in eliciting immune responses. These studies only involve cells with H-2b haplotype of MHC class I, while it is known that MHC class I molecules are downregulated on tumor cells, calling into question the potential efficacy of a peptide which binds primarily to these molecules. HLA polymorphisms among outbred human populations further restrict the efficacy of this peptide as a vaccine, if it only binds to specific haplotypes with high affinity. It makes sense to start with HLA-A*201, one of the most common human haplotypes, but other haplotypes must be looked into as well.
In the studies by Ressing et al where E7 11-20 proves to be poorly
immunogenic, more assays should be done to further characterize
all arms of the immune response against the peptide to help determine
if this avenue holds much promise or if it would be more worthwhile
to pursue other leads. As with all laboratory experiments, caution
must be exercised in generalizing results from in vitro assays
and small numbers of animals to what happens in the human body.
Importantly, the mice used are immunocompetent, while cervical
cancer patients may not be. It is unclear why Feltkamp et al,
after identifying 5 peptides in their initial study which bind
with high affinity to MHC class I molecules, do not test them
in mice but instead use previously identified peptides. Finally,
as noted by the authors, these peptides can only work in humans
if properly processed and presented by HPV 16-expressing cells
with H-2b haplotype and recognized by available CTL.
Vaccine adjuvants/carriers
The use of dendritic cells (DCs) as carriers for the putative CTL epitope E7 49-57 has also been explored as a way of enhancing immunogenicity without the use of external adjuvants such as IFA which may cause side effects in humans (15). DCs were selected to serve as 'professional' antigen-presenting cells based on studies showing the ability of tumor antigen-pulsed DCs to induce MHC class II-mediated specific immune responses in vitro and in vivo. Mice were immunized once or twice with a 2 week interval with either the E7 49-57 peptide in IFA, peptide-pulsed DCs, IFA alone, peptide in PBS, unpulsed DCs, or peptide-pulsed spleen cells. Tumor cell challenge occurred 2 weeks after the last immunization. A single immunization with peptide-pulsed DCs or peptide in IFA induced peptide-specific CD8+ lymphocyte responses in vivo and offered significant protection against tumor outgrowth, as compared to naive mice, although peptide in IFA protected a greater percentage of mice than did the peptide-loaded DCs. The authors concluded that peptide-loaded DCs can be used as potential tumor-specific vaccines because they are as immunogenic as peptide in IFA and can offer significant protection against tumors without the side effects which accompany IFA administration.
Comments. This study is well-designed and features a thorough
set of controls. However, tumor protection was more pronounced
in mice vaccinated with peptide in IFA than in mice vaccinated
with peptides carried by DCs, so DCs are not a clearly superior
adjuvant. Also, tumor growth in the mice is only followed for
60 days; further study is needed to determine if these results
hold out longer or if vaccine boosts are necessary and/or effective.
TraT, or Immunostimulatory Carrier (ISCAR), an integral membrane protein of E. coli, is known to help generate considerable antibody and T proliferative responses to peptides conjugated to it, and was therefore investigated as a adjuvant for HPV vaccines against cervical cancer (21). Two vaccines were created using HPV16 E7-derived peptides: BT5:ISCAR, containing a putative B epitope (peptides 44-48), Th epitope (peptides 48-54), and CTL epitope (peptides 49-57) conjugated to ISCAR, and BT17:ISCAR, containing only the B epitope conjugated to ISCAR. Immunization with BT5:ISCAR, but not ISCAR alone, induced IL-2 and IL-4 production. Immunization with BT5:ISCAR but not BT17:ISCAR induced specific CTL responses and offered in vivo protection against E7 containing tumor but not against non-E7 tumor. Both IgG2a (Th1-driven) and IgG1 (Th2-driven) antibody responses were detected in mice immunized with BT5:ISCAR or BT17:ISCAR but not BT5 or BT17 alone, and the authors conclude that these vaccines have the potential to elicit immune responses with a Th1 bias and invoke CTL responses without the use of adjuvants or recombinant vaccinia virus vector.
Comments. Theoretically, ISCAR holds promise as an adjuvant
for cervical cancer vaccines, but the data presented in this paper
leave many questions incompletely addressed. Appropriate negative
controls were used in each assay, but the mice used were free
of HPV 16 E7-containing tumors and were not immunocompromised
as might be patients who would potentially receive this vaccine.
Additionally, because antibody and CTL responses, IL-2 and IL-4
production, and protection against tumor cells elicited by immunization
were measured in different mice inoculated at different sites,
the overall effects of immunization in individual mice cannot
be ascertained. Only two peptides were tested, and only one or
sometimes two doses of vaccine were used in each assay, leaving
unexplored immune responses to other peptides, and the effects
of other concentrations of peptide:ISCAR conjugate.
Researchers have also studied the use of recombinant vaccinia virus (rVV) as a way to deliver HPV antigens to the immune system. Proper antigen processing and presentation require that the antigen be produced in host cells, which can happen if rVV carries the gene for the target antigen directly to the cells (3). Gao et al. compared the ability of rVV expressing intron-free HPV16 E6 sequence versus wild type vaccinia virus to elicit specific antibody, proliferative lymphocyte, and CTL responses in H-2b and H-2d mice (8). Boursnell et al. created a rVV vector expressing fused E6 and E7 sequences of HPV16 and 18 and compared its efficacy against wild type vaccinia and rVV expressing unmodified HPV16 E7 (3). To lessen the potential risk of oncogenesis by rVV, the authors used site directed mutagenesis on E7 to inactivate the Rb-binding site. Zhu et al. used H-2d mice, comparing the immunogenicity of rVV expressing HPV16 E7 peptides 1-52 or peptides 48-98 compared to E7 administered in the adjuvant MF59 (25).
In all three studies, the rVV vaccines induced specific Tc
and Th cell responses while the control vaccines did
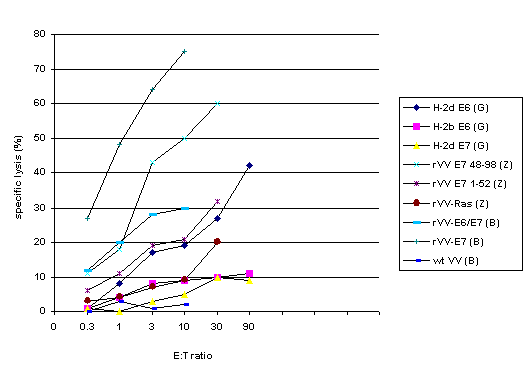
not; representative results are graphed in Figure 1.

Figure 1. CTL responses using recombinant
vaccinia virus vaccines.
The legend indicates the antigen
used to transfect target cells; cytotoxic activity of spleen cells
from mice immunized with the corresponding antigen is graphed.
References: G=Gao (8), B=Boursnell (3), Z=Zhu (25). Data from
the Zhu study show that immunization with E7-expressing rVV induced
more CTL activity than did E6/E7-expressing rVV; the authors attribute
this unexpected finding to reduced epitope density on cells infected
with E6/E7-expressing rVV.
While the results from these 3 studies are not completely comparable because different immunization protocols (dosage, number of vaccinations, intervals between vaccinations, haplotypes of mice tested) were employed, graphing them together shows the general trends clearly, with antigen-specific CTLs being induced and capable of target cell lysis in vitro following immunization with antigen-expressing rVV compared to immunization with wild type vaccinia virus and other controls.
Comments. Zhu et al. test vaccine with E7-expressing rVV against E7 in adjuvant, but do not have a control rVV that does not express peptide. Otherwise, these studies include the appropriate controls. Coordination among researchers could lead to more systematic testing of HPV antigens expressed in rVV vectors. Studies with rVV should progress to in vivo tumor protection or tumor eradication assays or even to human trials to yield data that will advance the field, rather than repeatedly demonstrating that rVV vaccines can induce cell-mediated immune responses. The vaccines used by Boursnell et al. were in fact used in the first published phase I/II trial, which is reviewed in a later section.
Lin et al. investigated another approach to enhance MHC class II antigen processing and presentation to CD4+ cells by linking HPV 16 E7 antigen to the sorting signals of lysosome-associated membrane protein (LAMP-1), creating a Sig/E7/LAMP-1 chimera (12). In initial studies, expression of this chimera in vitro and in vivo with a rVV vector succeeded in targeting E7 to endosomal and lysosomal compartments and enhanced antigen presentation compared to vaccinia expressing wild type E7 (24). This study tested these rVV vaccines (Sig/E7/LAMP-1 vaccinia, E7 vaccinia, and wild type vaccinia) for in vivo tumor protection and eradication of established tumors in mice. 80% of mice vaccinated with Sig/E7/LAMP-1 remained tumor-free 3 months after tumor cell injection, while all mice receiving either E7 or wild type vaccinia developed tumors within 3 weeks of tumor injection. A surprising lack of difference between E7 and wild type vaccinia was observed. Administration of Sig/E7/LAMP-1 following tumor injection also resulted in protection from tumor growth, while mice immunized with either of the other two vaccines showed progressive tumor growth within 2 weeks of tumor injection. Antibody depletion studies were also carried out, and indicated that CD4+, CD8+, and NK cell responses all played a role in antitumor immunity.
Comments. This novel approach yielded striking and encouraging
results, but as with previous studies, it would be preferred to
test these vaccines in humans now, or at least see the results
repeated by other labs using larger numbers of animals. Sig/E7/LAMP-1
vaccine is thought to enhance antigen presentation to CD4+ cells,
but it is widely believed that the CD8+ cell response is more
important in immune responses against tumors. What's important
is that the desired in vivo effects, tumor prevention and eradication,
are achieved, but it is also important to further study how these
effects are achieved using Sig/E7/LAMP-1, shedding more light
on the relative importance of CD4+ and CD8+ cell responses in
fighting HPV-induced tumors.
In comparing the different carriers and adjuvants used in the
above studies, no one strategy seems clearly superior to the others.
Because different carriers and adjuvants have not been tested
systematically with a range of different HPV peptides, the available
data are not complete enough allow any definitive conclusions
to be drawn. The individual strategies do have their advantages
and disadvantages, however. Oil-based mineral adjuvants, while
effect in animal studies, may cause side effects in humans, providing
impetus to experiment with other adjuvants. There are always safety
concerns surrounding the use of a "live" vaccine such
as recombinant vaccinia virus, but rVV has its advantages as well:
its genome can be manipulated to incorporate additional DNA, infection
with it induces an immune response, it can express a variety of
tumor-associated antigens, and it is lytic virus that doesn't
persist in cells or become latent so the possibility of an infected
cell surviving and becoming tumorigenic is small (3). Physiologic
carriers such as LAMP-1, ISCAR, and DCs should work in theory
and the results of these studies don't suggest otherwise, but
without an immunogenic, widely recognized, efficiently processed
epitope used as vaccine, it won't matter how effective the adjuvant
is.
Human trials
Human trials are the most important test of the efficacy of HPV 16 and 18 E6 and E7 proteins as immunotherapy for cervical cancer. To date, results from only one phase I/II trial have been published (2). This trial included eight late stage cervical cancer patients and used the recombinant vaccinia virus vaccine expressing fused E6 and E7 from HPV 16 and 18 constructed by Boursnell et al (3). 58 patients were initially screened to participate, but most were excluded due to immunocompromise as indicated by a low CD4 cell count. The vaccine appeared to be safe, causing no significant clinical side effects or environmental contamination and remaining stable after passage in vivo. All 8 patients mounted an antibody response against vaccinia, and 3/8 patients showed an HPV-specific antibody response attributable to vaccination. Only three patients were evaluable for HPV-specific CTLs, one of whom demonstrated a CTL response. Two patients remained tumor-free 15 and 21 months after immunization.
Comments. Now that this vaccine has been shown to be safe, at least so far, trials should be conducted on more patients with earlier stages of cervical cancer who are likely to be more immunocompetent. The exclusion of so many cancer patients because of immunocompromise raises real doubts about the efficacy of even a very good vaccine containing the most highly immunogenic HPV peptide. Further study should be focused on the two patients who remained tumor-free following vaccination, to monitor their survival and document their continuing immune responses over a longer period of follow up. Also, it would be interesting to know whether their tumor-free state is attributable to vaccination, and if so, what it is about their immune responses that allowed vaccination to be successful for them compared to the other patients. Overall, however, the results don't look exceedingly promising; this could be because the patients have such advanced disease, but at the same time this group of patients is most suitable for phase I/II trials because they don't have much to lose.
Other clinical trials are currently underway internationally and
in the US (sponsored by the NCI) with their results not yet published.
One Australian trial is using HPV16 E7 as a glutathione-S-transferase
(GST) bacterial fusion protein in Algammulin adjuvant, administered
3 times in escalating doses to 5 patients with late stage cervical
cancer (21). All 5 patients have produced antibodies to E7 and
2/3 evaluable subjects have shown proliferative T cell responses
to GST-E7 and E7 peptides. Another trial is using HPV16 E7 CTL
epitopes for HLA-A*201 has begun with patients who are unresponsive
to conventional therapies; patients have been vaccinated but no
data has been collected yet (21).
General critiques and future directions
It is difficult to conduct and critique cervical cancer vaccine research in a wholly unbiased way because we all want to see an effective vaccine that confers tumor protection or better yet, is able to eradicate established tumors. But before simply continuing to pursue a wide array of different ideas in a seemingly random fashion, researchers need to step back and gain some perspective. The idea of using immunotherapy against cancer is inherently flawed inasmuch as cancer patients are likely to have been immunocompromised in the course of aggressive conventional therapies. Additionally, tumors seem to have evolved very effective strategies for immune evasion, especially tumors of virally-induced cancers, making immunotherapy for cervical cancer an uphill battle from the start.
This is not to say that we shouldn't try, and researchers have indeed been trying, proceeding in logical steps that have allowed them to greatly increase their understanding of HPV and immunity. But this review of the literature suggests that efforts to develop an effective vaccine have become very narrowly focused. Table 1 shows that most groups are concentrating on HPV type 16 E7 protein, with several groups having studied peptides 49-57, the putative CTL epitope. Especially because the preliminary results of clinical vaccine trials don't look too promising, researchers might consider pursuing other avenues. For instance, a prophylactic vaccine for cervical cancer using HPV L1 surface antigen may be more promising than E6 and E7 therapeutic vaccines (16). Many scientific discoveries are purely serendipitous, and if everyone in the field is focused on E6 and E7 proteins, it may preclude other work that prove to be effective even though the avenues currently being pursued make the most sense theoretically. For instance, E4 and E5 could be better vaccine antigens than E6 and E7. Increased coordination between researchers in this field would also help to optimize resources and energy - not an unreasonable thought, considering that only a relatively small number of labs are intensely focused on this research.
A more detailed understanding of the precise immune mechanisms that are important in fighting HPV-induced cancers is necessary, for instance elucidating the role of NK cells, and the relative contributions of CD4 and CD8 cell-mediated responses (16). Another concern is that peptide vaccines may be too restricted in their HLA type and antigen specificity; there needs to be further investigation of vaccines which encode or harbor multiple epitopes, which may circumvent this problem (13). Heterogeneity is a problem not only with HLA, but also with viral epitopes, making exact matches between viral epitopes and HLA more difficult; this picture is further complicated by the possibility of mutation. A single amino acid substitution in an important binding region of a peptide or a slightly wrong peptide could induce T cell anergy instead of active stimulation in cervical cancer patients, and do more harm than good (20). Other experimental issues that remain to be addressed are 1) what vaccine dosages and how many boosts will be necessary to achieve optimal immunity using a potential vaccine, 2) whether peripheral tolerance and other forms of specific and nonspecific immunosuppression that may result from tumor growth, and 3) whether the longer duration of tumors in humans than in mice can increase tumor cell heterogeneity and affect antigen expression, processing , and presentation.
Many uncertainties and unanswered questions remain regarding the
use of HPV 16 and 18 E6 and E7 as immunotherapy for cervical cancer.
Research should continue in a more coordinated fashion, seeking
to further understand the intricacies of the immunology involved
in HPV-induced cancer and to be more comprehensive in addressing
different peptides and HLA types.
References
1. Acres B, Bizouarne N, Balloul JM, Kieny MP. Vaccine
immunotherapy of breast and cervical carcinoma: murine models.
http://perso.curie.fr/icoc/k.html
2. Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson
GWG, Westmoreland D, Evans AS, Adams M, Stacey SN, Boursnell MEG,
Rutherford E, Hickling JK, Inglis SC. A recombinant vaccinia virus
encoding human papillomavirus types 16 and 18, E6 and E7 proteins
as immunotherapy for cervical cancer. Lancet, 1996 Jun
1, 347(9014):1523-1527.
3. Boursnell MEG, Rutherford E, Hickling JK, Rollinson
EA, Munro AJ, Rolley N, McLean CS, Borysiewicz LK, Vousden K,
Inglis SC. Construction and characterisation of a recombinant
vaccinia virus expressing human papillomavirus proteins for immunotherapy
of cervical cancer. Vaccine 1996, 14(16): 1485-94.
4. Feltkamp MCW, Smits HL, Vierboom MPM, Minnaar
RP, de Jongh BM, Drijfhout JW, Schegget J, Melief CJM, Kast WM.
Vaccination with cytotoxic T lymphocyte epitope-containing peptide
protects against a tumor induced by human papillomavirus type
16-transformed cells. Eur. J. Immunol, 1993, 23:
2242-49.
5. Feltkamp MCW, Vreugdenhil GR, Vierboom MPM, Ras
E, van der Burg SH, Schegget J, Melief CJM, Kast WM. Cytotoxic
T lymphocytes raised against a subdominant epitope offered as
a synthetic peptide eradicate human papillomavirus type 16-induced
tumors. Eur. J. Immunol, 1995, 25: 2638-42.
6. Fields BN, Knipe DM, Howley PM, eds. Fields
Virology, third edition. Lippincott-Raven, Philadelphia, 1996.
7. Frazer IH. Immunology of papillomavirus infection.
Current Opinion in Immunology 1996, 8: 484-91.
8. Gao L, Chain B, Sinclair C, Crawford L, Zhou J,
Morris J, Zhu X, Stauss H. Immune response to human papillomavirus
type 16 E6 gene in a live vaccinia vector. Journal of General
Virology, 1994 Jan, 75 (Pt 1):157-64.
9. Gissmann L, Jochmus I, Nindl I, Muller M. Immune
response to genital papillomavirus infections in women. Prospects
for the development of a vaccine against cervical cancer. Annals
of the New York Academy of Sciences, 1993 Aug 12, 690:80-5.
10. Gissmann L. Immunologic responses to human papillomavirus
infection. Obstetrics and Gynecology Clinics of North America,
Sep 1996, 23(3): 625-39.
11. Glaser V. Companies intensify vaccine approach in battles against HIV and cancer. Genetic Engineering News, 1995 Feb 15. http://www.enews.com:80/data/magazines/ca...technology/tech/geng_
news/Archive/021595.3
12. Lin KY, Guarnieri FG, Staveley-O'Carroll KF,
Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established
tumors with a novel vaccine that enhances major histocompatibility
class II presentation of tumor antigen. Cancer Research,
1996 Jan 1, 56(1):21-6.
13. Melief CJM, Offringa R, Toes REM, Kast WM. Peptide-based
cancer vaccines. Current Opinion in Immunology 1996, 8:
651-57.
14. Meszaros L. Vaccine against HPV infection still
under development. Dermatology Times. http://www.modernmedicine.com:80/derm/std2834.html
15. Ossevoort MA, Feltkamp MCW, van Veen KJH, Melief
CJM, Kast WM. Dendritic cells as carriers for a cytotoxic T lymphocyte
epitope-based peptide vaccine in protection against a human papillomavirus
type 16-induced tumor. Journal of Immunotherapy 1995, 18(2):
86-94.
16. Palefsky J. personal communication, 3/13/97.
17. Ressing ME, Sette A, Brandt RMP, Ruppert J, Wentworth
PA, Hartman M, Oseroff C, Grey HM, Melief CJM, Kast WM. Human
CTL epitopes encoded by human papillomavirus type 16 E6 and E7
identified through in vivo and in vitro immunogenicity studies
of HLA-A*0201-binding peptides. The Journal of Immunology,
1995, 154(11): 5934-43.
18. Ressing ME, van Driel WJ, Celis E, Sette A, Brandt
RMP, Hartman M, Anholts JDH, Schreuder GMT, ter Harmsel WB, Fleuren
GJ, Trimbos BJ, Kast WM, Melief CJM. Occasional memory cytotoxic
T-cell responses of patients with human papillomavirus type 16-positive
cervical lesions against a human leukocyte antigen-A*0201-restricted
E7-encoded epitope. Cancer Research, Feb 1 1996, 56:
582-8.
19. Restifo NP. The new vaccines: building viruses
that elicit antitumor immunity. Current Opinion in Immunology
1996, 8: 658-63.
20. Stern PL. Immunity to human papillomavirus-associated
cervical neoplasia. Advances in Cancer Research 1996, 69:
175-211.
21. Tindle, RW, Croft S, Herd K, Malcom K, Geczy
AF, Stewart T, Fernando GJP. A vaccine conjugate of 'ISCAR' immunocarrier
and peptide epitopes of the E7 cervical cancer-associated protein
of human papillomavirus type 16 elicits specific Th1- and Th2-type
responses in immunized mice in the absence of oil-based adjuvants.
Clin. Exp. Immunol. 1995, 101: 265-71.
22. Tindle RW. Human papillomavirus vaccines for
cervical cancer. Current Opinion in Immunology 1996, 8:
643-50.
23. van Driel WJ, Ressing ME, Brandt RMP, Toes REM,
Fleuren GJ, Trimbos JB, Kast WM, Melief CJM. The current status
of therapeutic HPV vaccine. Annals of Medicine 1996, 28:
471-77.
24. Wu T, Guarnieri FG, Staveley-O'Carroll KF, Viscidi
RP, Levitsky HI, Hedrick L, Cho KR, August JT, Pardoll DM. Engineering
an intracellular pathway for major histocompatibility complex
class II presentation of antigens. Proc. Natl. Acad. Sci. USA,
Dec. 1995, 92: 11671-75.
25. Zhu X, Tommasino M, Vousden K, Sadovnikava E, Rappuoli R, Crawford L, Kast M, Melief CJM, Beverley PCL, Stauss HJ. Both immunization with protein and recombinant vaccinia virus can stimulate CTL specific for the E7 protein of human papillomavirus 16 in H-2d mice. Scand. J. Immunol 1995, 42: 557-63.